ELREXFIO™ Shows Over Two-Year Median Survival in Relapsed/Refractory Multiple Myeloma
Pfizer Inc. revealed comprehensive overall survival data from the Phase 2 MagnetisMM-3 trial assessing ELREXFIO™ (elranatamab-bcmm) in patients with significantly pretreated relapsed or refractory multiple myeloma. Findings from the study indicated a median OS of 24.6 months for cohort A in this essential single-arm study.
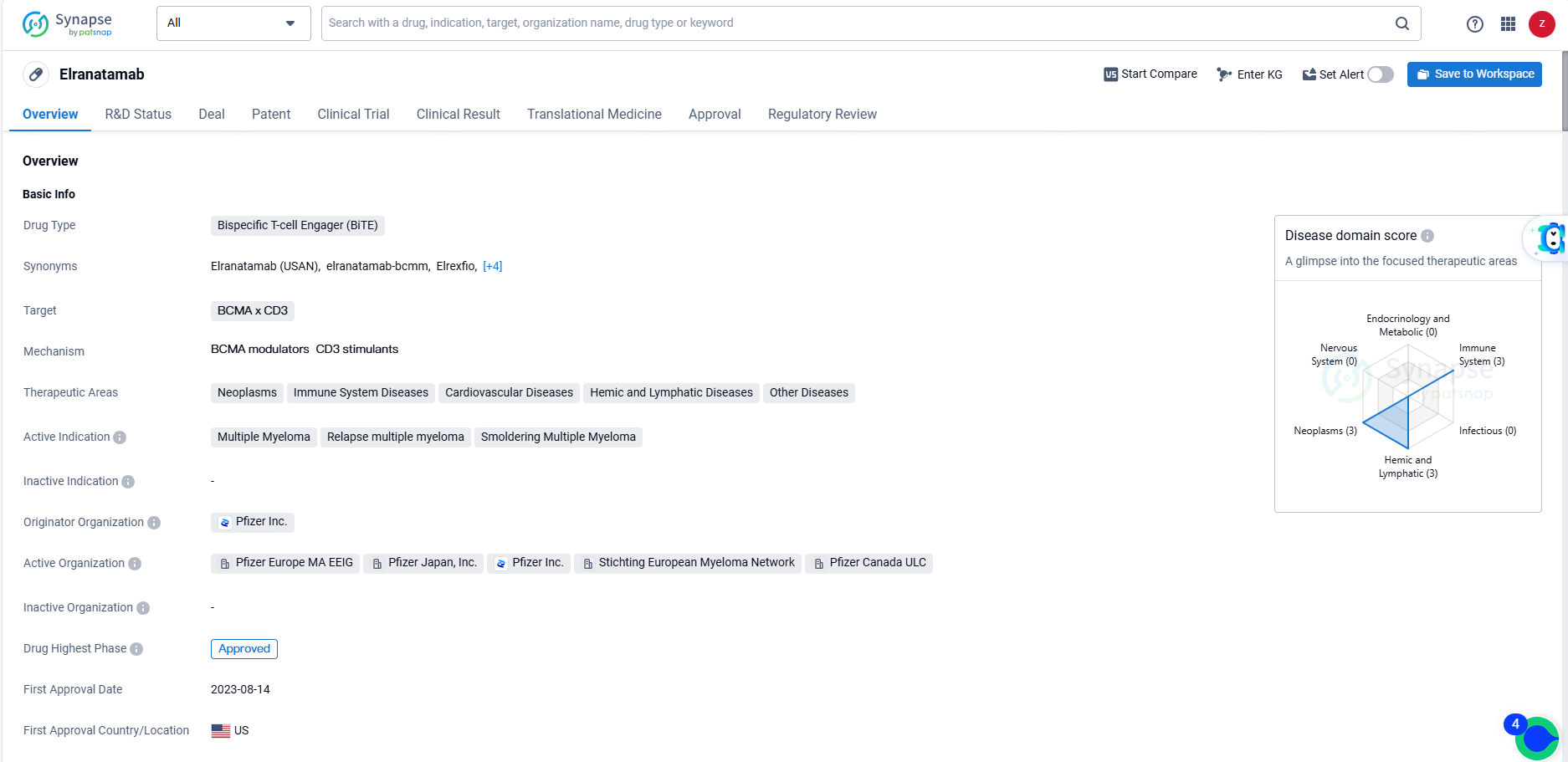
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
The data from MagnetisMM-3 will be showcased in a poster session at the European Hematology Association Hybrid Congress, happening in Madrid, Spain, from June 13-16. Additionally, presentations at EHA 2024 will feature ELREXFIO data spanning the comprehensive MagnetisMM clinical trial series.
“These promising overall survival figures underscore the clinical advantage ELREXFIO has already displayed and its potential to become a game-changing treatment option for individuals with multiple myeloma,” stated Roger Dansey, M.D., Chief Development Officer, Oncology, Pfizer.
“Patients with relapsed or refractory multiple myeloma often face limited treatment options as their disease advances due to resistance to therapy, leading to progressively shorter remission and response durations,” noted MagnetisMM-3 clinical trial investigator Mohamad Mohty, M.D., Ph.D., Professor of Hematology and Head of the Hematology and Cellular Therapy Department at Saint-Antoine Hospital and Sorbonne University, Paris, France. “These noteworthy overall survival data are especially encouraging given the severely advanced patient cohort with characteristics linked to poorer outcomes.”
Pfizer’s comprehensive MagnetisMM clinical development program is evaluating the use of elranatamab across the full spectrum of MM patients, from RRMM to newly diagnosed cases. Current ongoing registrational-intent trials are pitting elranatamab against current standards of care both as monotherapy and in combination with standard or new therapies.
These trials include MagnetisMM-4, studying elranatamab alongside other anti-cancer therapies, MagnetisMM-5 in double-class exposed environments, MagnetisMM-6 in newly diagnosed patients ineligible for stem cell transplants, MagnetisMM-7 in newly diagnosed patients post-transplant, and MagnetisMM-32 in individuals who have previously undergone anti-CD38-directed therapy.
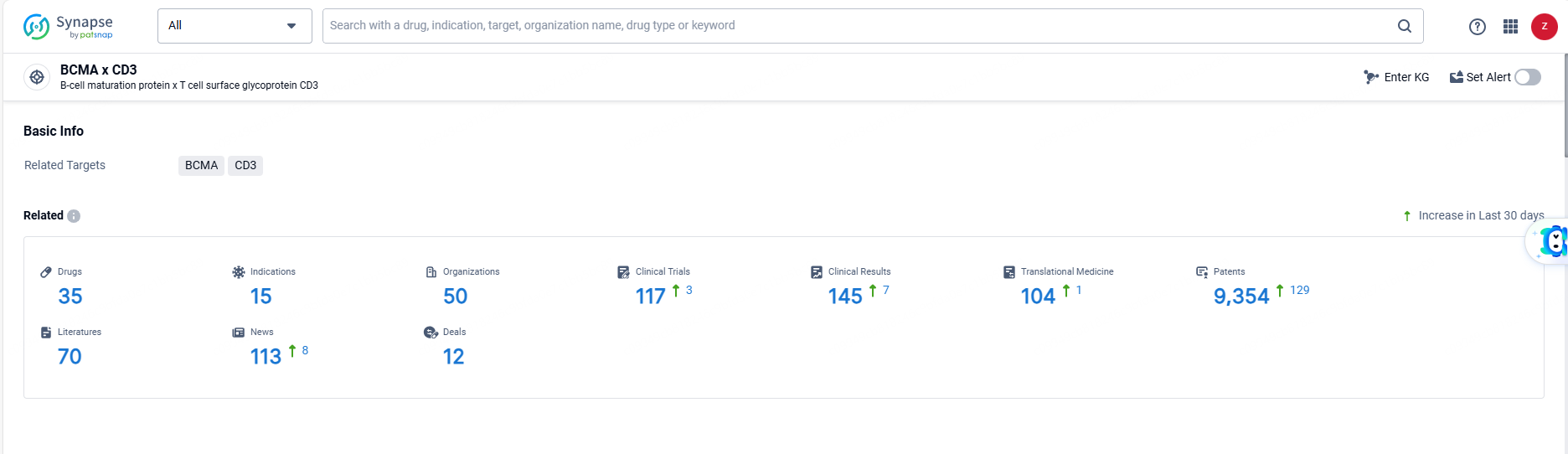
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of June 18, 2024, there are 35 investigational drugs for the BCMA and CD3 targets, including 15 indications, 50 R&D institutions involved, with related clinical trials reaching 117, and as many as 9354 patents.
Elranatamab is a bispecific T-cell engager drug developed by Pfizer Inc. It targets BCMA x CD3 and is indicated for multiple myeloma, relapse multiple myeloma, and smoldering multiple myeloma. The drug has received various regulatory designations and is expected to receive its first approval in the United States in August 2023. Additionally, it has reached the highest phase of approval globally and is currently in the NDA/BLA phase.