Celltrion USA Launches Cost-Effective Adalimumab Biosimilar as a Budget-Friendly Alternative to HUMIRA®
Celltrion USA has declared the availability of their adalimumab-aaty, a high-concentration (100 mg/mL) and citrate-free biosimilar to HUMIRA (adalimumab), at a reduced wholesale acquisition price.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
Adalimumab-aaty is set to be marketed at a WAC (Wholesale Acquisition Cost) list price, and it will be available at a substantial 85% lower price compared to the current WAC list price of HUMIRA. Additionally, under the brand name YUFLYMA, Celltrion USA offers Adalimumab-aaty. Since its market introduction in July 2023, YUFLYMA can be purchased at a 5% discounted rate from the existing WAC list price of HUMIRA.
This medication is sanctioned for use in managing eight specific medical conditions, including rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and hidradenitis suppurativa.
Thomas Nusbickel, the Chief Commercial Officer at Celltrion USA, stated, “Navigating medication access in the U.S. is increasingly intricate. The introduction of biosimilars into the market brings considerable value by fostering competition, and branded as well as non-branded forms of adalimumab-aaty are set to enhance the availability and affordability of adalimumab biosimilars across the U.S., offering financial gains for both consumers and the healthcare sector at large.”
Adalimumab-aaty is the non-branded variant of YUFLYMA, a fully human recombinant anti-TNFα monoclonal antibody. YUFLYMA has received FDA approval for treating various ailments including rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, Crohn’s disease, ulcerative colitis, plaque psoriasis, and Hidradenitis Suppurativa. Alongside the July 2023 release of YUFLYMA in dosages of 40mg/0.4mL, and a subsequent 80mg/0.8mL version in December 2023, an additional 20mg/0.2mL dosage was introduced in March 2024 in the U.S.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
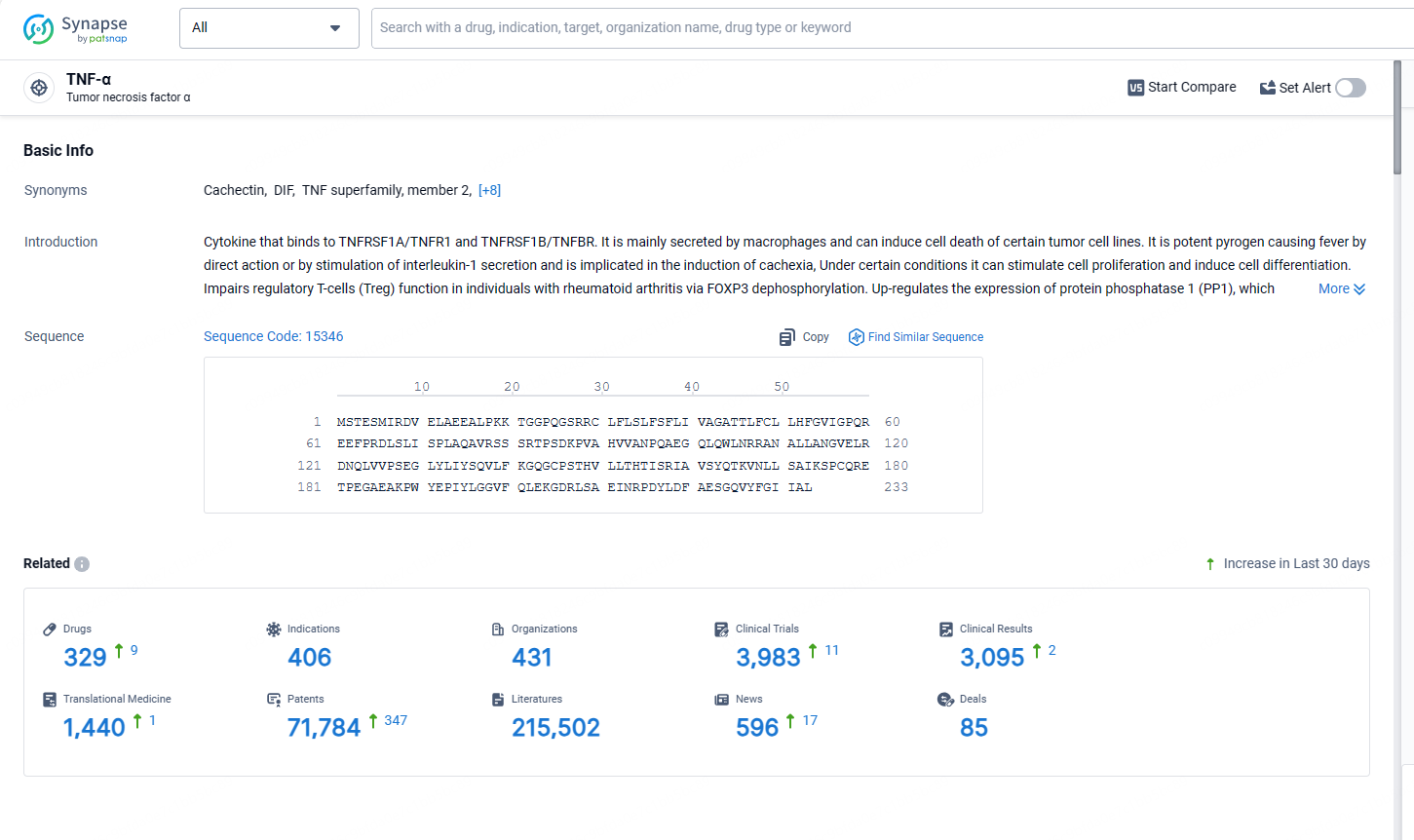
According to the data provided by the Synapse Database, As of May 13, 2024, there are 329 investigational drugs for the TNF-α target, including 409 indications, 431 R&D institutions involved, with related clinical trials reaching 3983, and as many as 71784 patents.
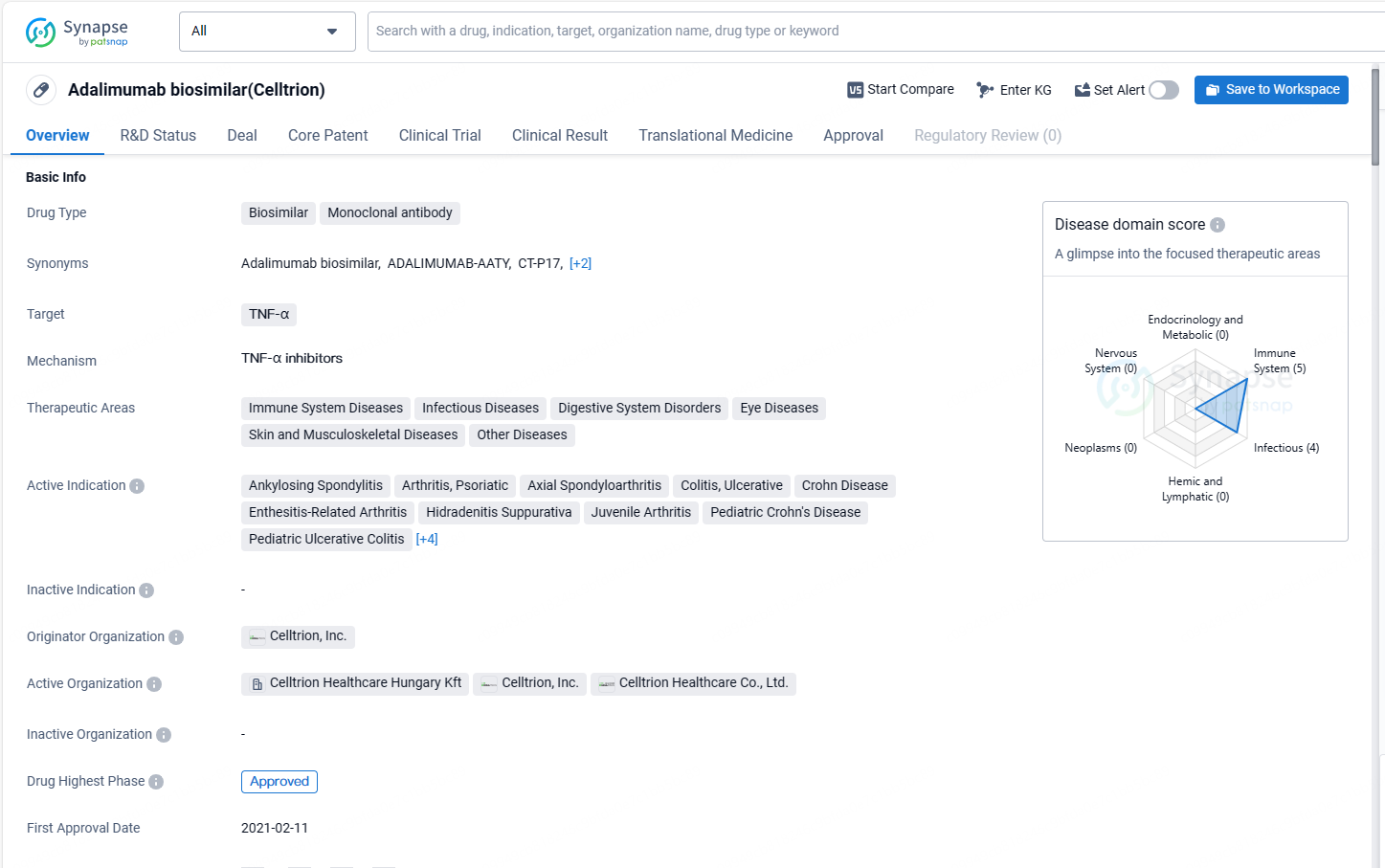
Adalimumab-aaty targets TNF-α and has been approved for use in various therapeutic areas, including immune system diseases, infectious diseases, digestive system disorders, eye diseases, skin and musculoskeletal diseases, and other diseases. The drug has a wide range of active indications, covering conditions such as arthritis, colitis, psoriasis, and uveitis. Its first approval was obtained in February 2021 in the European Union, Iceland, Liechtenstein.