X4 Pharmaceuticals, focused on therapeutic opportunities targeting the CXCR4 receptor
X4 Pharmaceuticals is a biotech company specialized in the development of therapeutic drugs focusing on CXCR4 and immunological biology. The company maintains its professional strengths at the corporate headquarters in Boston, USA, and its center of excellence in Vienna, Austria, continuously driving forward its patient-centered mission. X4 is advancing treatment developments for patients with rare immunological diseases and groups with significant unmet medical needs by researching and promoting innovative therapies.
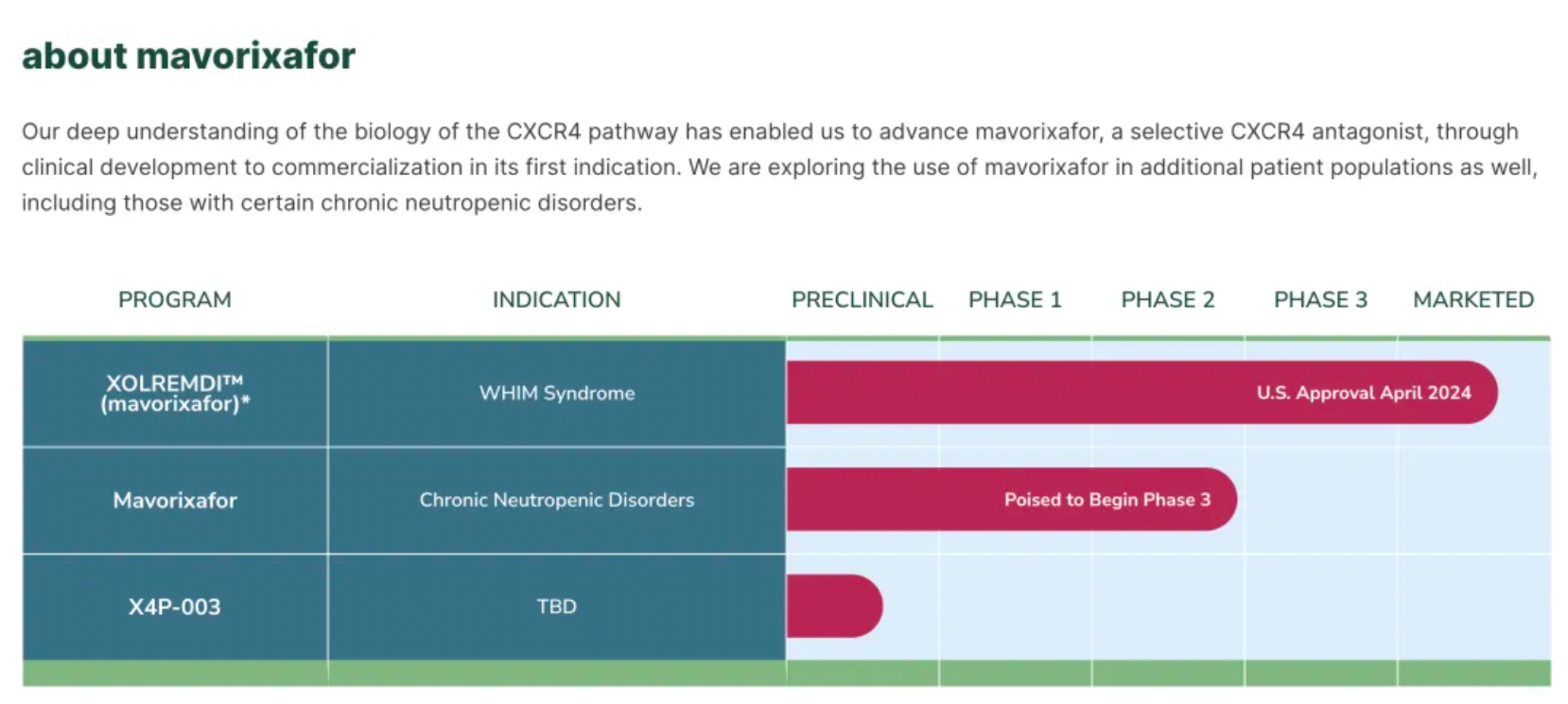
On April 29th, X4 Pharmaceuticals announced that the U.S. FDA had approved XOLREMDI™ (Mavorixafor) capsules for the treatment of patients aged 12 and older with WHIM syndrome, to increase the number of mature neutrophils and lymphocytes in the circulation. WHIM syndrome is a rare, congenital immune deficiency disorder and chronic neutropenia characterized by warty lesions, hypogammaglobulinemia, susceptibility to infections, and bone marrow retention of neutrophils.
Currently, the company is working to expand the application of Mavorixafor to a broader patient population and is about to launch a pivotal global Phase III clinical trial targeting certain patients with chronic neutropenia.
The following is the company presentation material updated in April 2024 by X4 Corporation:
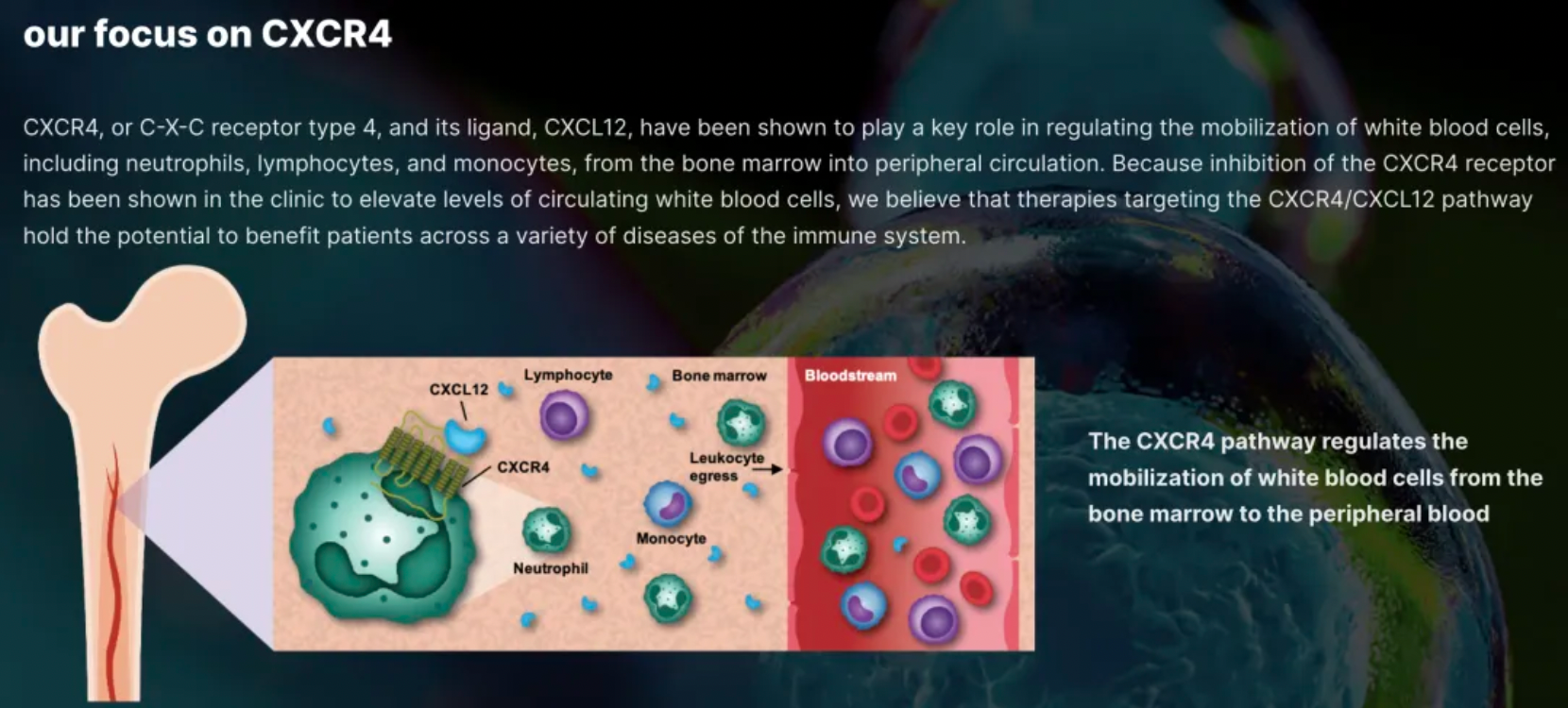
X4 Corporation is a typical biotechnology company focused on the biology and clinical drug development targeting a single receptor, CXCR4.

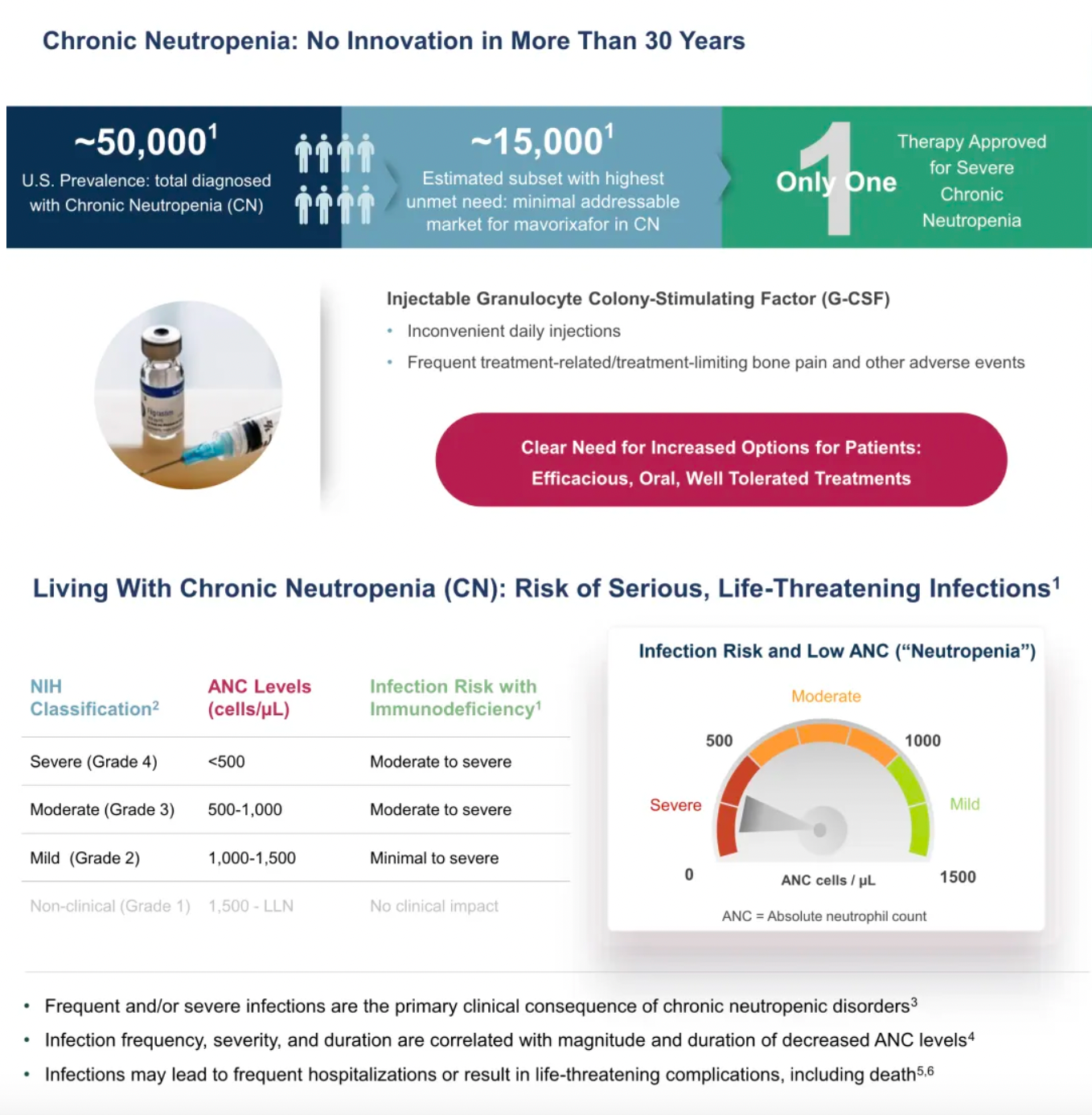
There has been no innovative drug development progress for chronic neutropenia over the past 30 years.
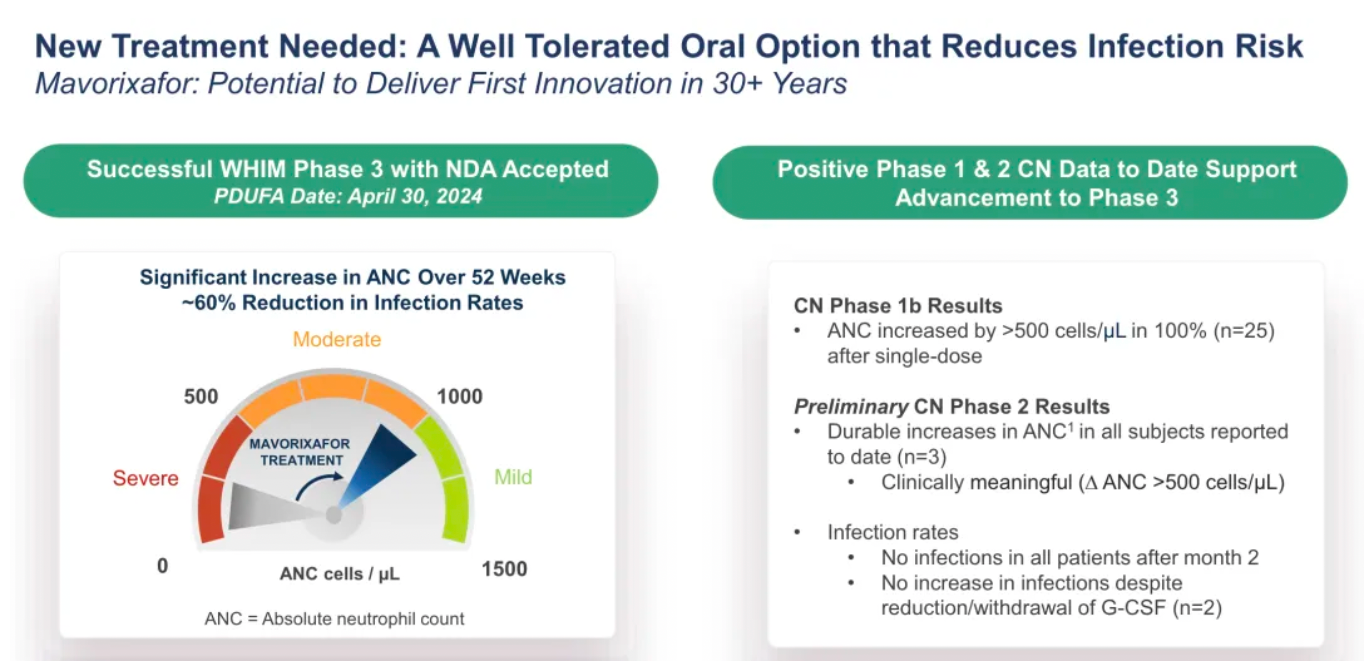
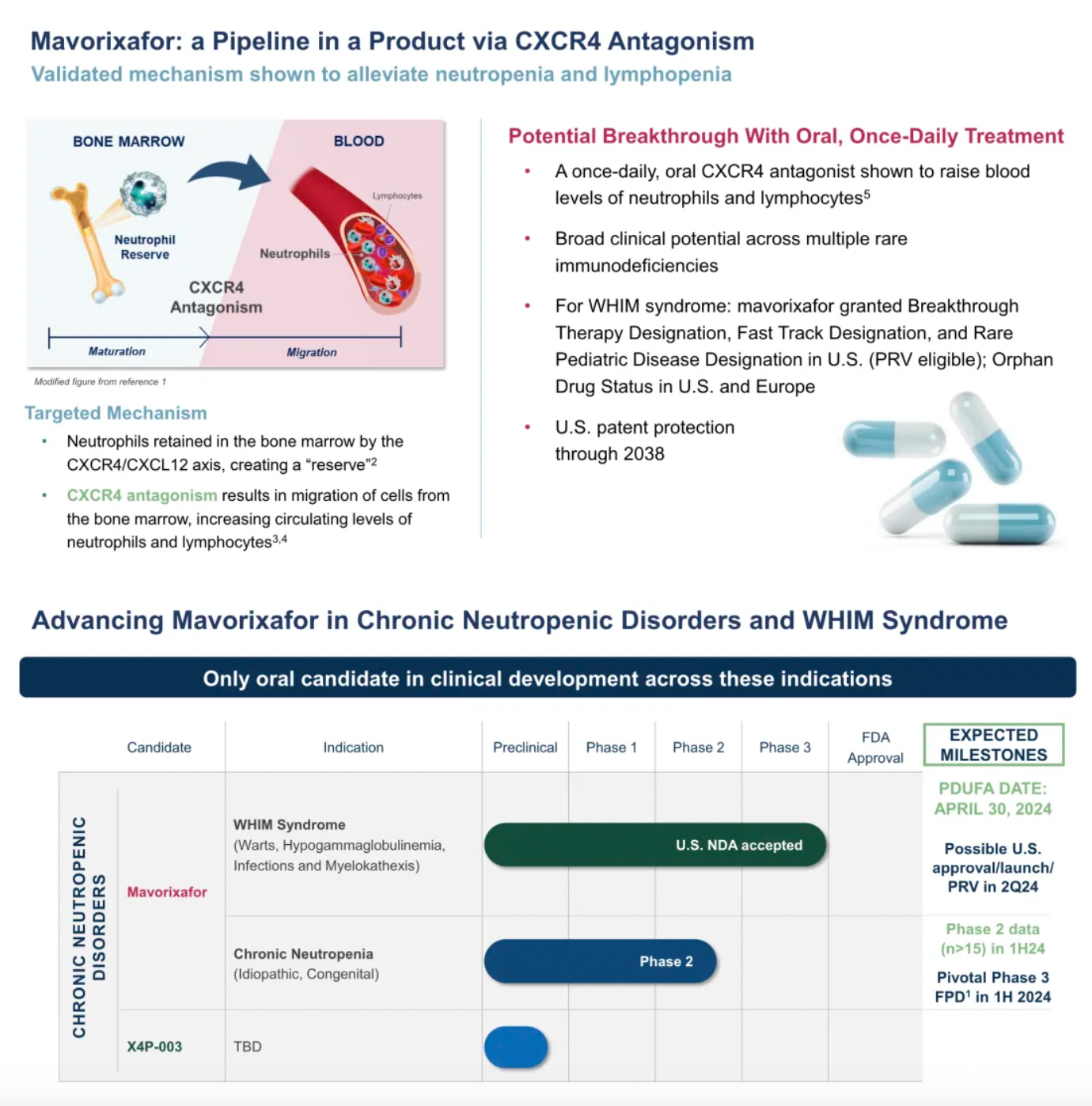
Mavorixafor: Verified to be effective in alleviating neutropenia and lymphocytopenia.
For more information, please click the image below to access the Synapse database.