CheckMate-227 Trial Phase 3 confirms prolonged survival with Opdivo and Yervoy in Metastatic Non-Small Cell Lung Cancer
Bristol Myers Squibb released the results of six years from the initial part of the phase 3 CheckMate-227 study. It persistently exhibits the lasting and stable survival perks of Opdivo(nivolumab) in combination with Yervoy(ipilimumab) as opposed to chemotherapy in the primary treatment for the patients suffering from metastatic non-small cell lung cancer, irrespective of their PD-L1 expression status.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The subsequent results will be highlighted in a verbal discourse and have been designated for the official news program at the IASLC 2023 World Conference on Lung Cancer sponsored by the International Association for the Study of Lung Cancer on September 11, 2023.
The physicians noticed no unexpected safety signals and found the safety record of the combined immunotherapy pair Opdivo and Yervoy to be reliable, consistent with previously shared information from the same trial, and manageable within approved guidelines.
“Immunotherapy has transformed how we approach advanced lung cancer treatments. Now, for many patients, a diagnosis doesn't have the same implications it once had. These results from six years of tracking show us consistent and incredibly long-term clinical survival advantages when using nivolumab along with ipilimumab year after year,“ remarks Solange Peters, M.D., Ph.D., the chair and educator of medical oncology.
“We are ecstatic to see that Opdivo plus Yervoy is maintaining almost twice the survival rates compared to chemotherapy after six years of monitoring – the longest-ever for a Phase 3 trial with immunotherapy in metastatic non-small cell lung cancer. Our future focus is broadening our exploration into targeted and tiny molecule treatments, alongside additional immunotherapy pairing, in hopes of discovering possible solutions for as many thoracic cancer patients as we can.”
Opdivo combined with Yervoy - centered mixtures have displayed substantial enhancements in Overall Survival in six Phase 3 clinical studies across five different types of tumors so far, which includes metastatic NSCLC, metastatic melanoma, advanced renal cell carcinoma malignant pleural mesothelioma, and esophageal squamous cell carcinoma.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
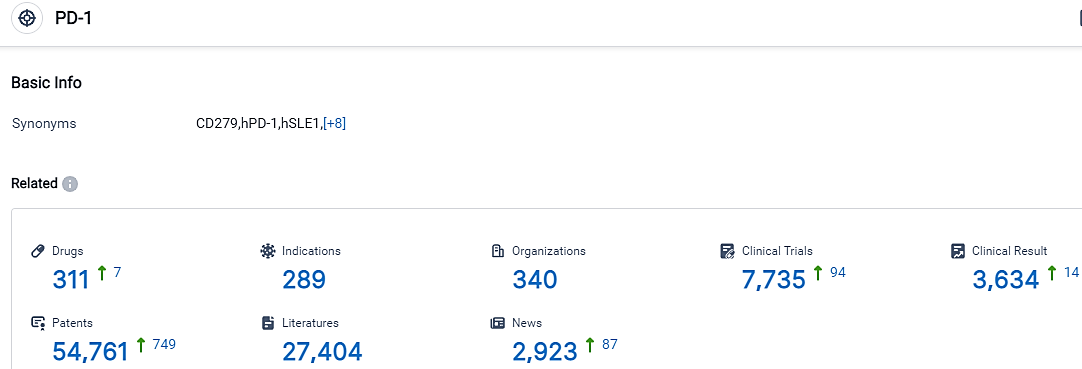
According to the data provided by the Synapse Database, As of September 15, 2023, there are 311 investigational drugs for the PD-1 target, including 289 applicable indications,340 R&D institutions involved, with related clinical trials reaching 7735,and as many as 54761 patents.
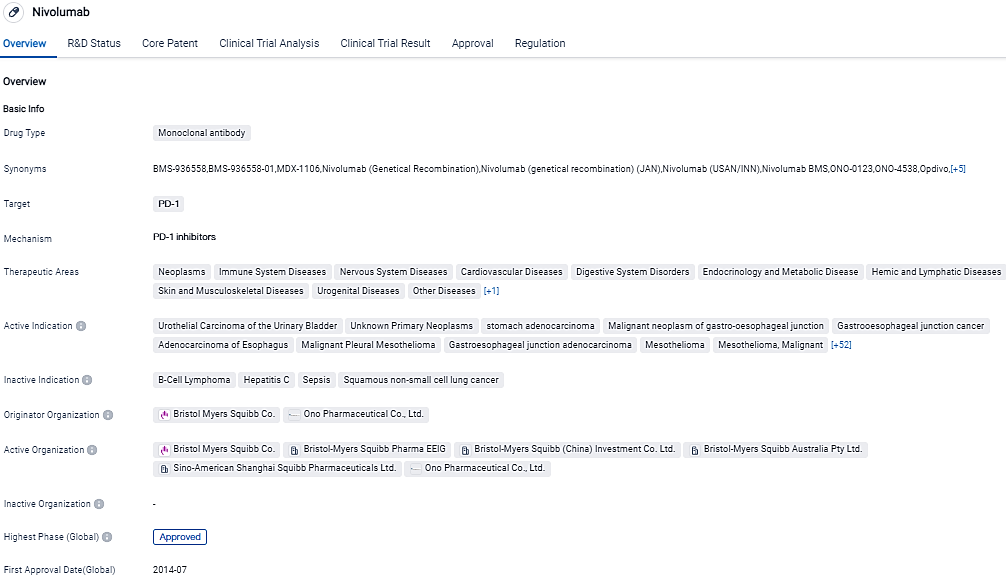
In July 2014, Opdivo was the first PD-1 immune checkpoint inhibitor to receive regulatory approval anywhere in the world. Opdivois currently approved in more than 65 countries, including the United States, the European Union, Japan and China. In October 2015, the Company’s Opdivo and Yervoy combination regimen was the first Immuno-Oncology to receive regulatory approval for the treatment of metastatic melanoma and is currently approved in more than 50 countries, including the United States and the European Union.