CheckMate-8HW: Opdivo & Yervoy Combo Reduces Disease Progression or Fatality by 79% in MSI-H/dMMR Metastatic Colorectal Cancer Patients Compared to Chemotherapy
Bristol Myers Squibb released data from the third-phase clinical study CheckMate -8HW, which assessed the efficacy of combining Opdivo (nivolumab) with Yervoy (ipilimumab) versus a standard chemotherapy selected by the investigator. This was done in the context of initial treatment for individuals diagnosed with metastatic colorectal cancer that is characterized by high microsatellite instability (MSI-H) or a deficiency in mismatch repair (dMMR).
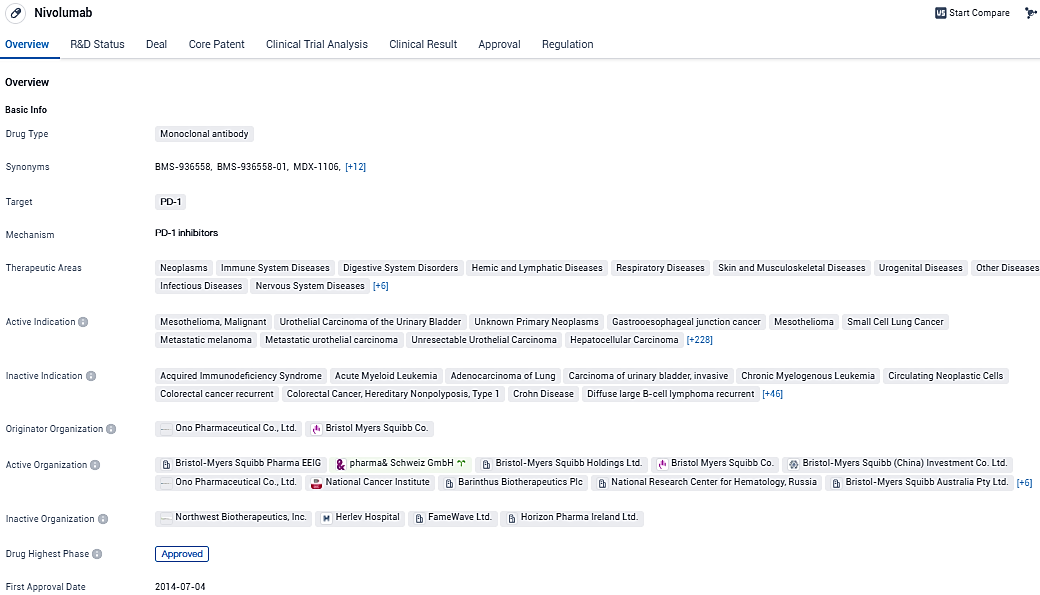
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The combined immunotherapeutic strategy using Opdivo along with Yervoy has shown significant and substantial improvements in the primary measurement of progression-free survival (PFS). This was determined by an objective Blinded Independent Central Review, registering a 79% diminishing in the likelihood of disease worsening or patient mortality as compared with conventional chemotherapy in subjects with centrally verified MSI-H/dMMR mCRC.
Signs of enhanced PFS was apparent starting around the three-month mark and continued over time. The median PFS has not been reached for the group receiving Opdivo and Yervoy, whereas it was 5.9 months for those on chemotherapy. This advantage in PFS was consistently seen across various specified patient groups, regardless of KRAS or NRAS mutations or the existence of metastases in organs like the liver, lungs, or peritoneum at the start of the treatment.
The safety and tolerability profile of the Opdivo-Yervoy regimen aligns with what was previously described in the literature, and it was effectively managed using standard protocols without detecting any novel safety concerns. Higher-grade (grade 3/4) side effects tied to the treatment were reported in 23% of the group on the dual therapy and 48% in the chemotherapy group. The rate of discontinuing treatment due to any adverse events related to treatment was 17% in the combined therapy group versus 32% in the chemotherapy group.
Dr. Thierry Andre from the Medical Oncology Department at Sorbonne University and Hospital Saint-Antoine in Paris, France stated, “Patients with MSI-H/dMMR mCRC often do not respond sufficiently to chemotherapy. The nivolumab and ipilimumab regimen, compared to chemotherapy, provided a prominent PFS enhancement, observable from as early as three months. The findings exemplify the considerable effectiveness of this dual-therapy, offering a transformative approach for treating individuals in this clinical setting.”
Opdivo, categorized as a PD-1 immune checkpoint inhibitor, has been crafted to leverage the body's immune defenses and restore the immune system's ability to recognize and combat tumor cells. With its ability to activate the immune system against cancer, Opdivo plays a critical role in the management of various cancer types.
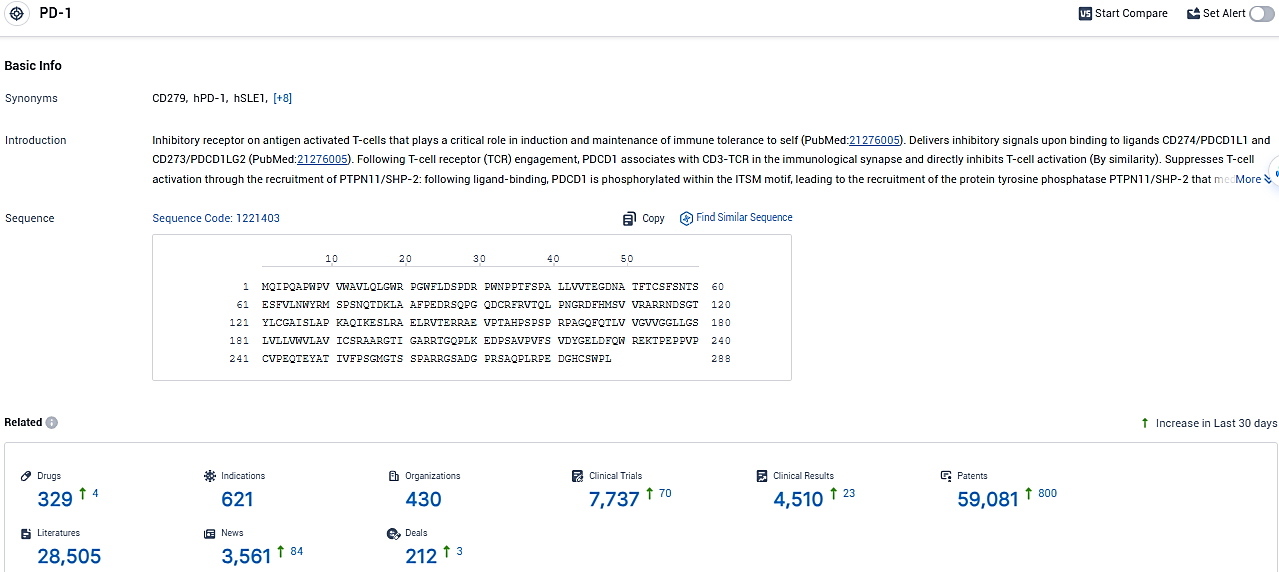
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 26, 2024, there are 329 investigational drugs for the PD-1 target, including 621 indications, 430 R&D institutions involved, with related clinical trials reaching 7737, and as many as 59081 patents.
In July 2014, Opdivo was the first PD-1 immune checkpoint inhibitor to receive regulatory approval anywhere in the world. Opdivo is currently approved in more than 65 countries, including the United States, the European Union, Japan and China. In September 2015, the Company’s Opdivo and Yervoy combination regimen was the first Immuno-Oncology combination to receive regulatory approval for the treatment of metastatic melanoma and is currently approved in more than 50 countries, including the United States and the European Union.