Chemomab Therapeutics Reveals Successful Phase 2 Study Outcomes: CM-101 Meets Key Targets
Chemomab Therapeutics Ltd., a biotech firm at the clinical stage dedicated to creating groundbreaking treatments for fibro-inflammatory conditions with significant unmet medical needs, announced encouraging initial outcomes from the Phase 2 SPRING study. This trial evaluated their pioneering monoclonal antibody, CM-101, in individuals suffering from primary sclerosing cholangitis.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
 Administration of CM-101 successfully reached its primary endpoint of ensuring safety and tolerability, while also displaying notable anti-fibrotic, anti-inflammatory, and anti-cholestatic properties across multiple disease-related secondary efficacy measures. These measures included a statistically significant reduction in liver stiffness, an essential marker of PSC disease. CM-101 emerges as the premier investigational drug in development for PSC to exert comprehensive, clinically significant effects on all three main disease components, reinforcing its multifaceted mechanism and potential for disease modification.
Administration of CM-101 successfully reached its primary endpoint of ensuring safety and tolerability, while also displaying notable anti-fibrotic, anti-inflammatory, and anti-cholestatic properties across multiple disease-related secondary efficacy measures. These measures included a statistically significant reduction in liver stiffness, an essential marker of PSC disease. CM-101 emerges as the premier investigational drug in development for PSC to exert comprehensive, clinically significant effects on all three main disease components, reinforcing its multifaceted mechanism and potential for disease modification.
In the clinical trial, CM-101 not only met the primary endpoints but also achieved key secondary objectives, being the inaugural therapy to show comprehensive, clinically relevant impacts on the three primary facets of PSC. Remarkably, this trial is the first among PSC studies to demonstrate a statistically notable decrease in liver stiffness within just 15 weeks of treatment, utilizing a widely accepted and validated measure for evaluating PSC progression. Additionally, CM-101 is one of the pioneering investigational therapies to reduce total bilirubin, a critical cholestasis marker indicative of liver health, along with decreased pruritus, a significant cholestatic symptom for patients.
“We are elated to announce the positive outcomes of the Phase 2 SPRING trial, representing a pivotal achievement for Chemomab and establishing clear clinical proof-of-concept for CM-101 in PSC and potentially other fibrotic ailments,” said Adi Mor, PhD, co-founder, CEO, and Chief Scientific Officer of Chemomab. “These findings robustly support progressing CM-101 to a Phase 3 PSC trial, which we aim to initiate in 2025 following our discussions with the FDA later this year. I extend my gratitude to the patients, caregivers, investigators, clinical staff, and patient advocacy groups, whose dedication and effort were invaluable.”
The primary endpoint of the study was met by CM-101, showing a positive safety profile throughout the 15-week treatment duration. Patients treated with CM-101, who had moderate/advanced disease, exhibited improvements across a broad spectrum of disease-related secondary endpoints. These included assessments of changes from baseline relative to placebo at Week 15 in liver stiffness, liver fibrosis biomarkers such as the Enhanced Liver Fibrosis score and PRO-C3 levels, total bilirubin, liver function tests, pruritis indicators, and markers of inflammation.
CM-101 demonstrated a positive safety and tolerability profile during the 15-week treatment period, with favorable and dose-dependent pharmacokinetic profiles. Adverse events, including fatigue, headache, and pruritis, were generally mild/moderate and similarly distributed between the placebo and CM-101-treated groups.
Chemomab is planning an End-of-Phase 2 meeting with the FDA to discuss the SPRING trial results and outline a proposed Phase 3 PSC trial aimed at accelerated approval. The company expects to complete these discussions by the year's end and receive official written feedback from the FDA in the first quarter of 2025.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
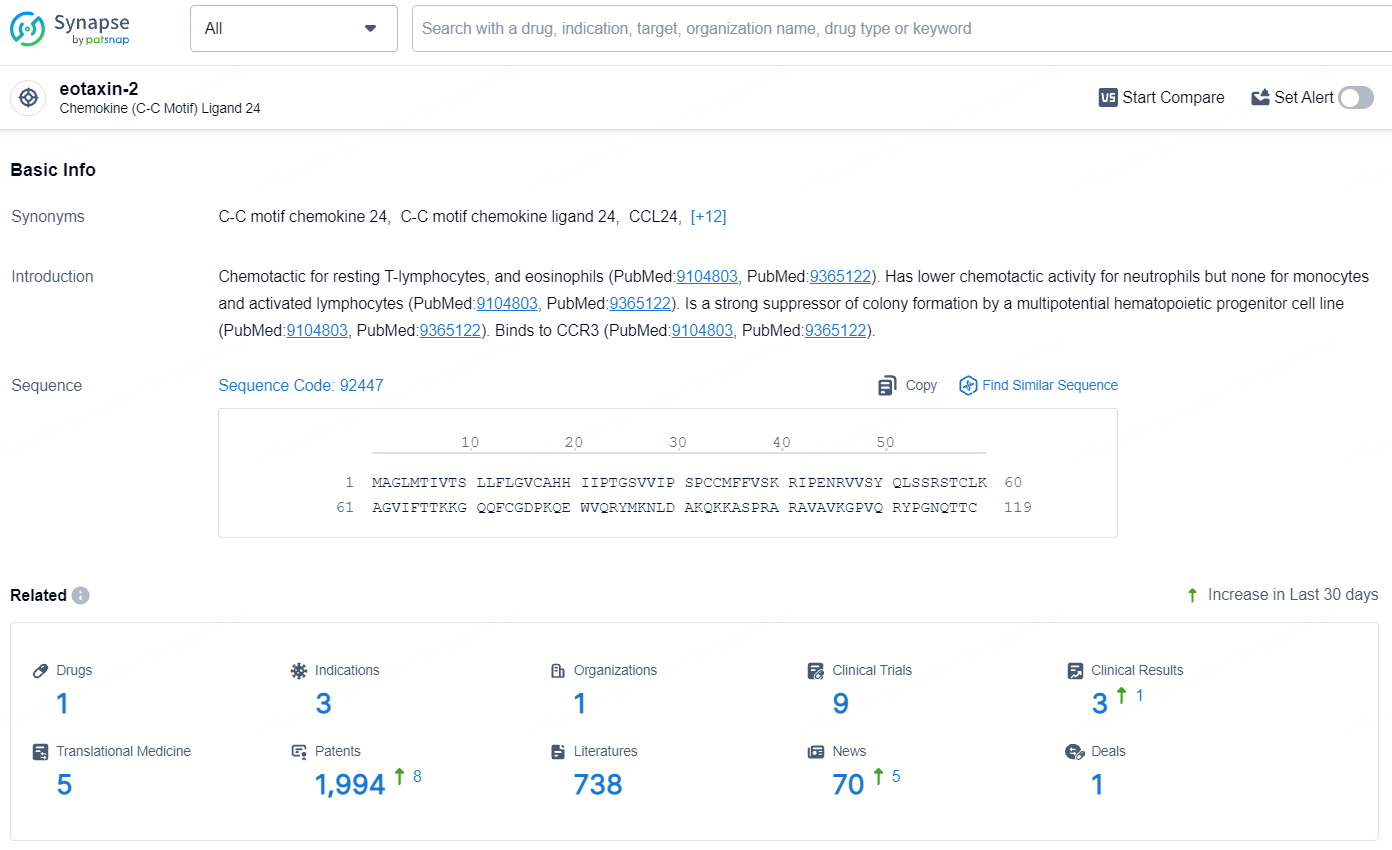
According to the data provided by the Synapse Database, As of July 31, 2024, there are 1 investigational drug for the eotaxin-2 target, including 3 indications, 1 R&D institution involved, with related clinical trials reaching 9, and as many as 1994 patents.
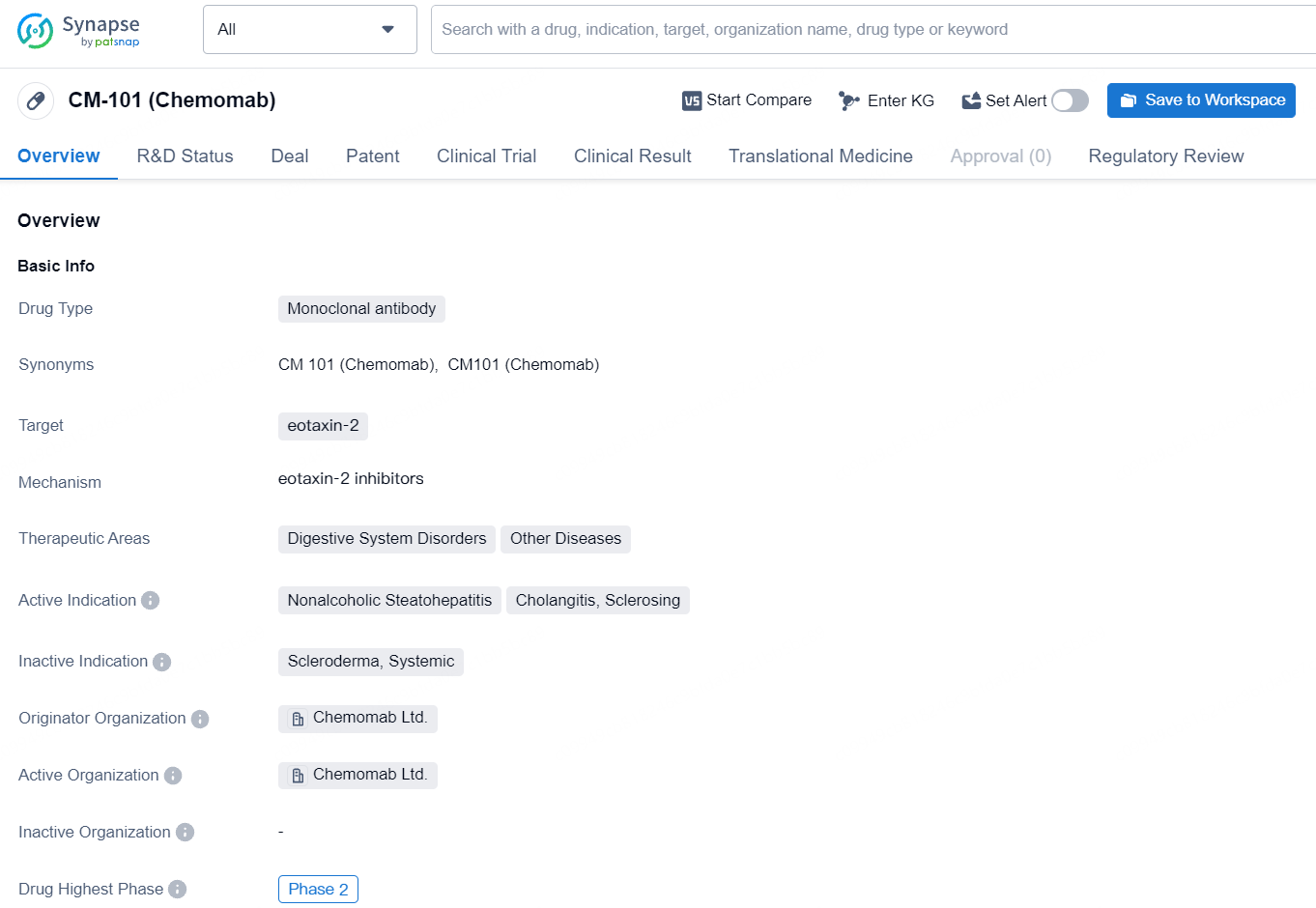
CM-101 specifically targets eotaxin-2, a protein involved in inflammatory responses. By targeting this protein, the drug aims to modulate the immune response and potentially alleviate symptoms and slow the progression of the targeted diseases. CM-101 represents a significant effort in addressing digestive system disorders and other related diseases, with a focus on Nonalcoholic Steatohepatitis, Cholangitis, and Sclerosing. Its advancement to Phase 2, coupled with regulatory designations, underscores its potential impact, although further clinical data and regulatory milestones will ultimately shape its future prospects in the pharmaceutical market.