Is Omaveloxolone approved by the FDA?
Yes, Omaveloxolone, marketed under the brand name Skyclarys, is FDA approved. The FDA approved Skyclarys on February 28, 2023, for the treatment of Friedreich's ataxia in adults and teenagers 16 years and older.
Indications:
Skyclarys (omaveloxolone) is indicated for:
- The treatment of Friedreich's ataxia, a rare, inherited disease that causes progressive damage to the nervous system resulting in symptoms ranging from gait disturbance and speech problems to heart disease. It is approved for use in adults and adolescents aged 16 years and older.
How Does Omaveloxolone Work?
Omaveloxolone is designed to activate the nuclear factor (erythroid-derived 2)-like 2 (Nrf2) pathway, which helps protect against oxidative stress and inflammation, mechanisms implicated in the pathology of Friedreich's ataxia.
Usage Instructions:
- Dosage: The recommended dose is 150 mg taken orally on an empty stomach once daily.
- Administration: Take omaveloxolone at least 1 hour before a meal. Swallow the capsule whole without crushing, chewing, breaking, or opening it.
- Monitoring: Regular blood tests are required to monitor for any adverse effects or necessary adjustments.
Side Effects:
Common side effects of omaveloxolone may include:
- Nausea
- Stomach pain
- Diarrhea
- Abnormal lab tests
- Headache
- Tiredness
- Muscle pain
Serious side effects requiring immediate medical attention include:
- Heart problems such as swelling, rapid weight gain, or feeling short of breath
- Signs of an allergic reaction like hives, difficulty breathing, and swelling of the face, lips, tongue, or throat
Patients experiencing any severe side effects should contact their healthcare provider promptly. Side effects can be reported to the FDA at 1-800-FDA-1088.
Warnings and Precautions:
- Heart Issues: Call your doctor at once if you experience swelling, rapid weight gain, or shortness of breath.
- Pregnancy and Birth Control: Omaveloxolone can make hormonal contraceptives less effective. Patients using hormonal birth control should discuss alternative methods with their doctor, such as condoms or non-hormonal intrauterine devices, while using omaveloxolone and for at least 28 days after the last dose.
- Pre-existing Conditions: Patients should inform their doctor if they have a history of heart failure, high cholesterol, liver disease, or any other significant medical condition.
- Grapefruit Interaction: Grapefruit products may interact with omaveloxolone and cause side effects, so they should be avoided.
Drug Interactions:
Several drugs can interact with omaveloxolone, including but not limited to:
- Nefazodone
- St. John's wort
- Antibiotics (e.g., clarithromycin, rifampin)
- Antifungals (e.g., itraconazole, ketoconazole)
- Antivirals for HIV or hepatitis C (e.g., boceprevir, cobicistat)
- Cancer medicines (e.g., apalutamide, enzalutamide)
- Seizure medicines (e.g., carbamazepine, phenytoin)
- Steroid medicines (e.g., dexamethasone, prednisone)
Patients should inform their doctor of all medications they are currently taking, including prescription, over-the-counter medicines, vitamins, and herbal products, to avoid potential drug interactions.
Conclusion:
Skyclarys (omaveloxolone) is an FDA-approved oral medication for the treatment of Friedreich's ataxia in individuals aged 16 and older. Approved on February 28, 2023, it offers a significant therapeutic option for managing this debilitating condition. Patients prescribed this medication should adhere to their healthcare provider's instructions and be mindful of potential side effects and drug interactions.
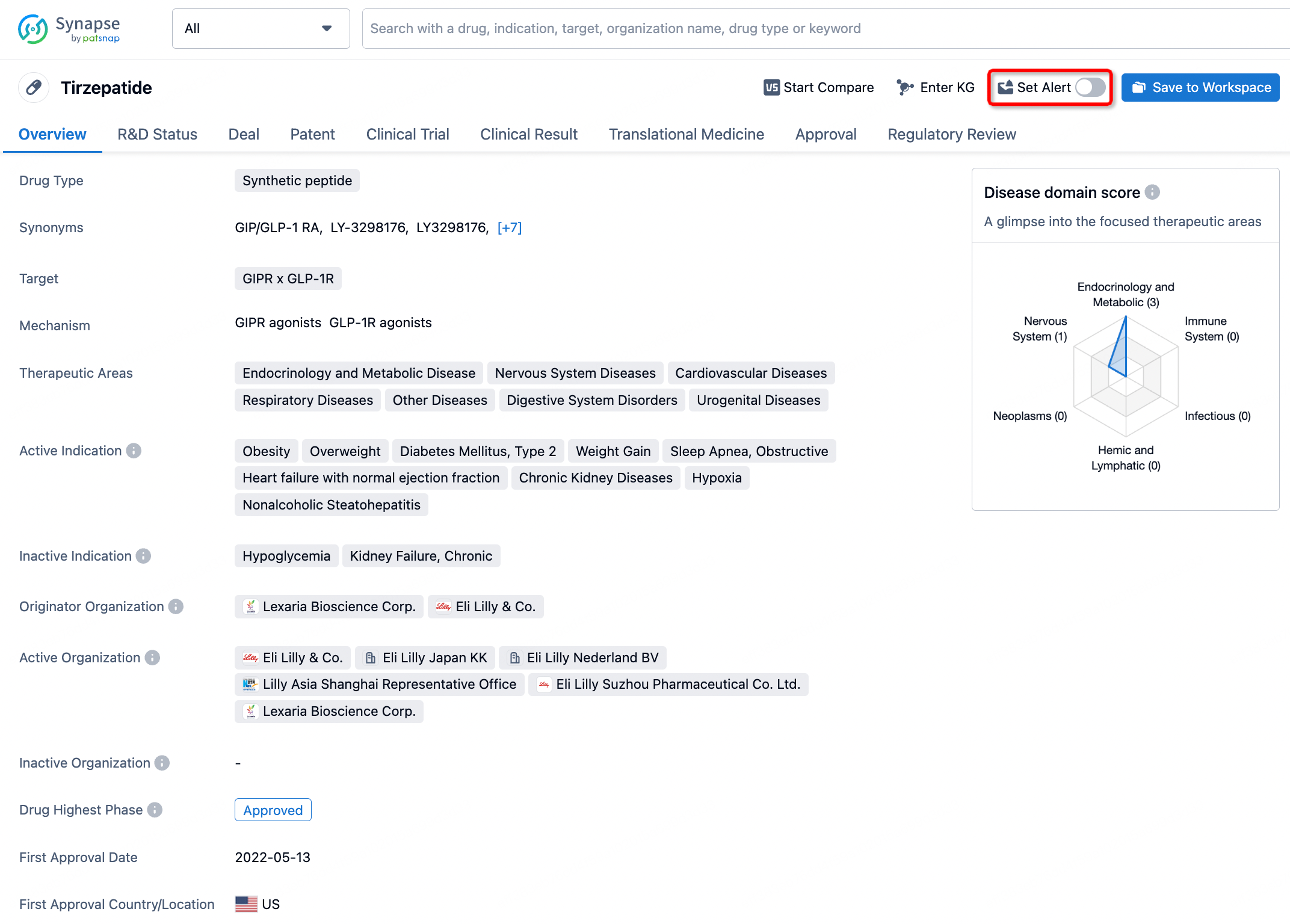
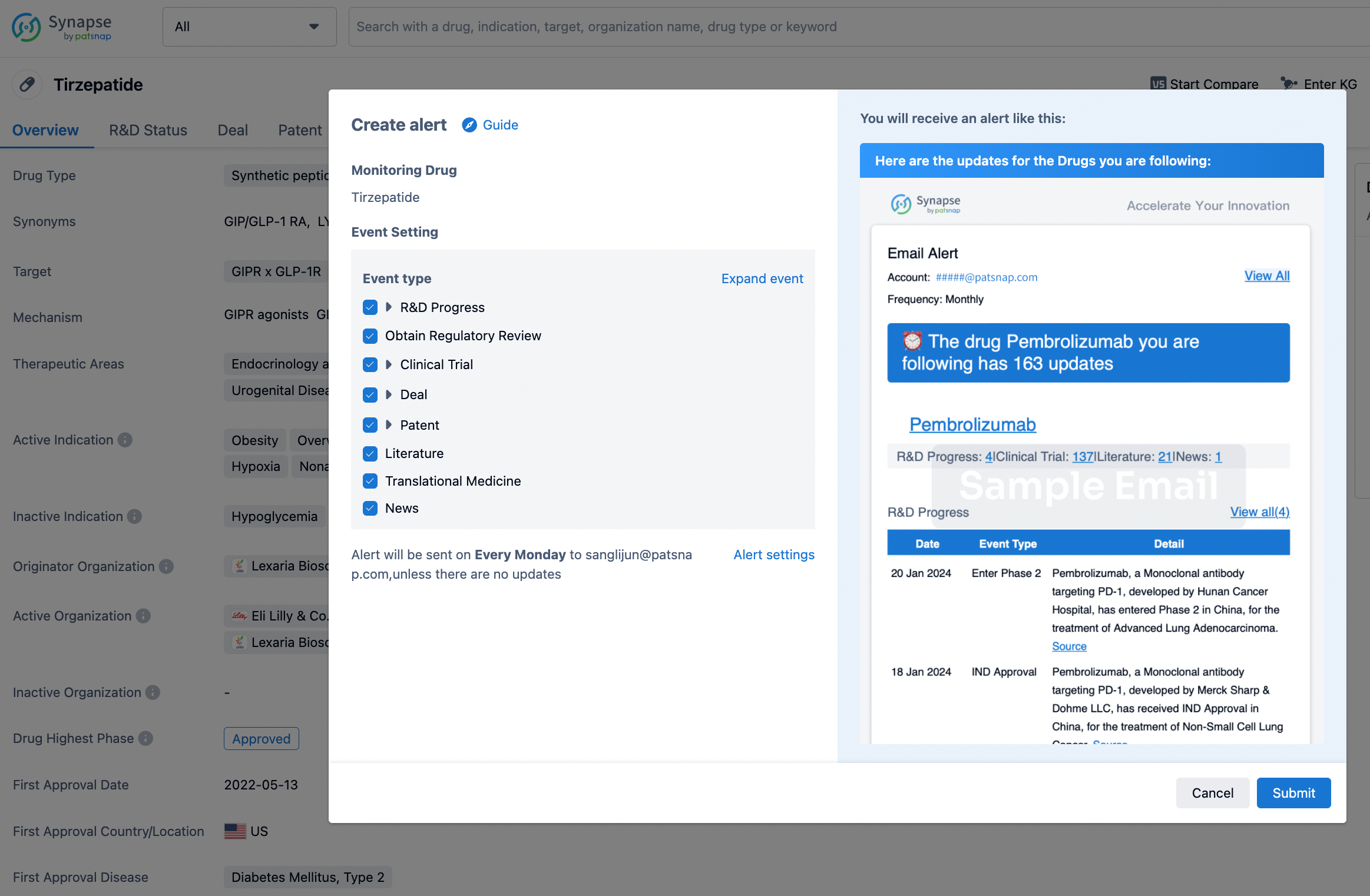
How to obtain the latest development progress of all drugs?
In the Synapse database, you can stay updated on the latest research and development advances of all drugs. This service is accessible anytime and anywhere, with updates available daily or weekly. Use the "Set Alert" function to stay informed. Click on the image below to embark on a brand new journey of drug discovery!