FDA Approves BioMarin's BRINEURA® for CLN2 Disease in Children Under 3
BioMarin Pharmaceutical Inc. revealed that the U.S. Food and Drug Administration has granted approval for its supplemental Biologics License Application concerning BRINEURA (cerliponase alfa). This approval aims to decelerate the rate of ambulation loss in children, regardless of age, who suffer from neuronal ceroid lipofuscinosis type 2, also referred to as tripeptidyl peptidase 1 (TPP1) deficiency.
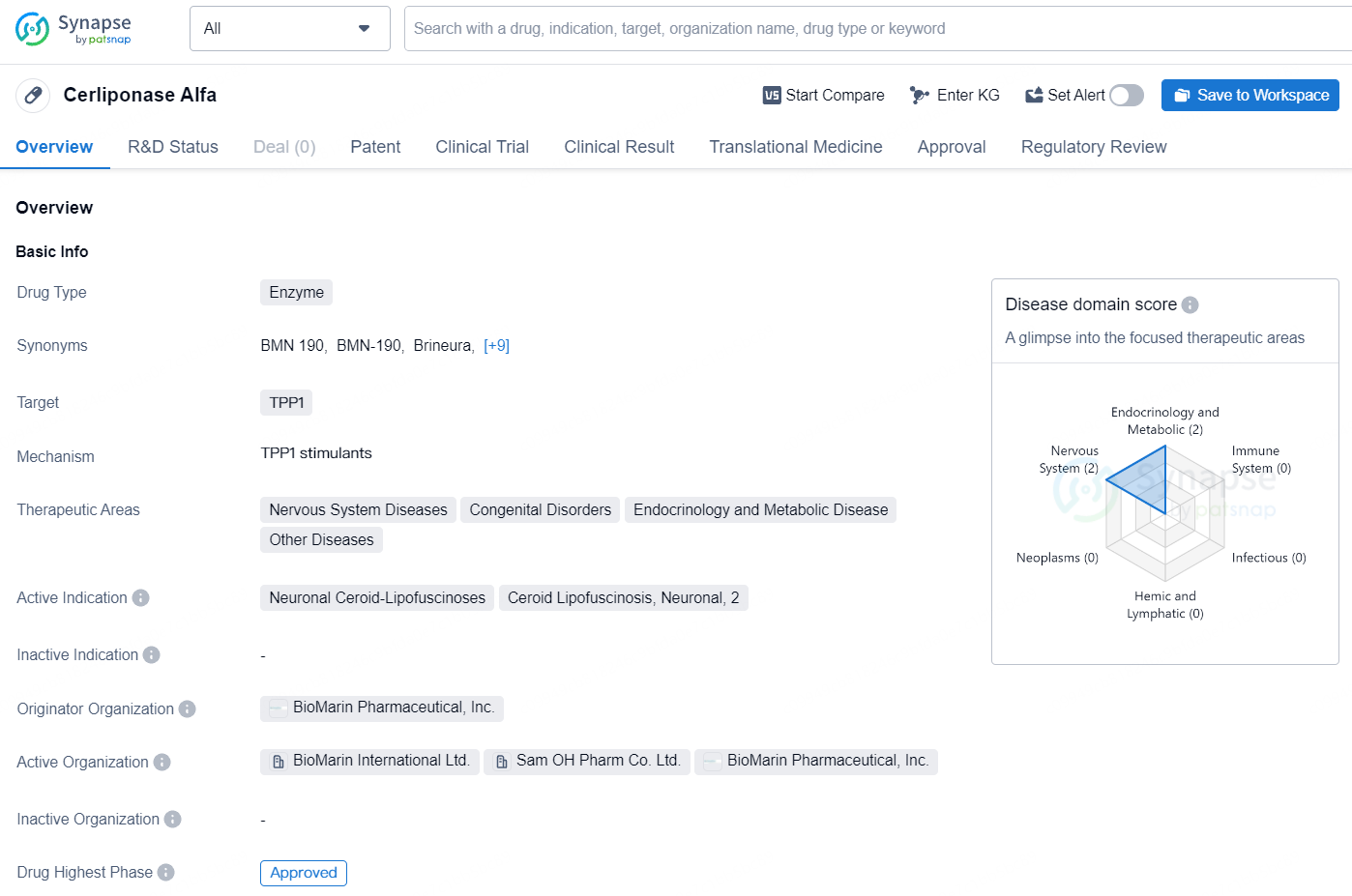
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
Previously, BRINEURA was recommended for children aged 3 years and above who exhibited symptoms of late infantile CLN2 disease. The updated indication now encompasses children of all ages with CLN2 disease, whether they show symptoms or are presymptomatic.
"The approval marks a crucial advancement in our ability to treat children with BRINEURA at the earliest possible stage, maximizing our potential to alter the disease's progression," said Hank Fuchs, M.D., president of Worldwide Research and Development at BioMarin. "Time is of the essence for families grappling with severe genetic disorders like CLN2 disease, which is known for its swift onset of neurodegenerative symptoms. Since BRINEURA’s initial approval, we have diligently worked to facilitate its expanded application for children of all ages, even those not yet exhibiting symptoms."
The supplemental Biologics License Application (sBLA) is underpinned by data from Study 190-203, a Phase 2, open-label, multicenter trial that assessed the efficacy of BRINEURA over approximately three years in children aged 1-6 years at the start, including eight children younger than 3 years. Findings from Study 190-203, presented at the 20th Annual WORLDSymposium in February, indicated that ICV-administered BRINEURA decelerated motor function decline and postponed disease onset in children with CLN2 disease, including those under 3 years of age.
BRINEURA’s safety profile is well-documented, with safety outcomes in children younger than 3 years consistent with the known safety profile of the drug. Besides confirming that initiating treatment after 3 years old significantly slows the progression of CLN2 disease, this is the first evidence showing that starting treatment before the age of 3 may delay the onset of the disease.
BRINEURA is an enzyme replacement therapy aimed at slowing the loss of walking or crawling abilities in pediatric patients with neuronal ceroid lipofuscinosis type 2, also referred to as tripeptidyl peptidase 1 (TPP1) deficiency, a variant of Batten disease.
BRINEURA is a recombinant form of human TPP1, the deficient enzyme in children with CLN2 disease, designed to reinstate TPP1 enzyme activity and degrade the accumulated storage materials causing CLN2 disease. For it to reach the brain and central nervous system cells, the treatment is administered directly into the cerebrospinal fluid using BioMarin’s proprietary technology.
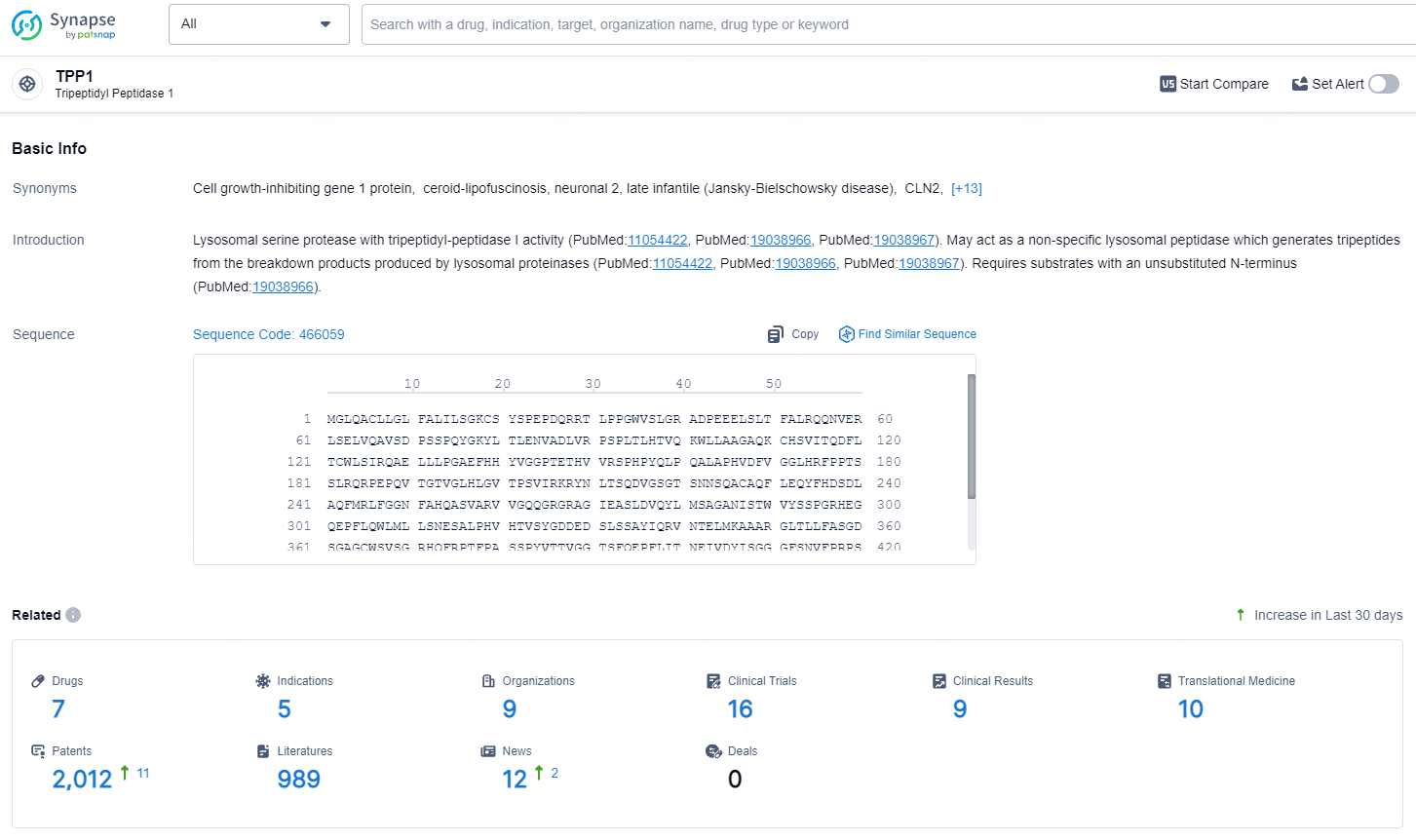
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of July 31, 2024, there are 7 investigational drugs for the TPP1 target, including 5 indications, 7 R&D institutions involved, with related clinical trials reaching 16, and as many as 2012 patents.
Cerliponase Alfa's approval and regulatory status position it as a valuable intervention for addressing unmet medical needs in various therapeutic areas, particularly in the treatment of rare and debilitating diseases. Its development represents a positive advancement in the pharmaceutical industry, offering hope for patients and healthcare providers seeking effective treatments for these complex conditions.