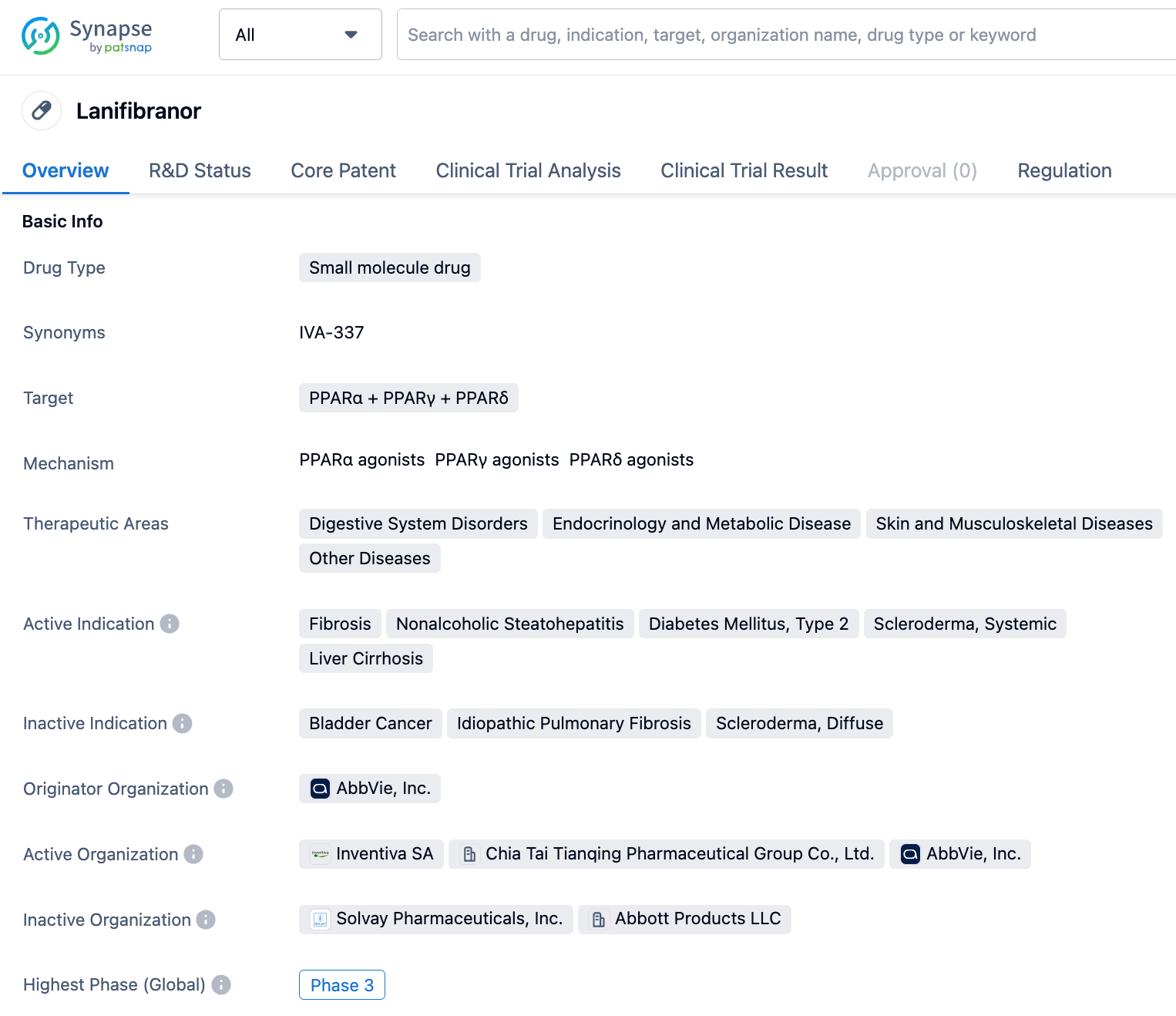
Chia Tai Tianqing Pharmaceutical initiates global multicenter phase III clinical trials for the pan-PPAR agonist Lanifibranor
On September 11, 2023, Chia Tai Tianqing Pharmaceutical registered a global multi-center phase III clinical trial. This trial is designed to evaluate the effectiveness and safety of Lanifibranor in treating adults with non-alcoholic steatohepatitis (NASH) which is accompanied by stage 2/3 (F2/F3) hepatic fibrosis without cirrhosis.
Lanifibranor is an orally taken pan-PPAR agonist developed by Inventiva. It exhibits moderate agonistic activity on three PPAR (α/γ/δ), thereby inducing anti-fibrotic, anti-inflammatory, and other beneficial vascular and metabolic changes in the body. On September 22, 2022, Sino Biopharmaceutical announced that on September 21, 2022, Chia Tai Tianqing, a subsidiary of the group, formally signed a licensing agreement with Inventiva S. A. (Inventiva). The agreement is to develop, produce, and commercialize Lanifibranor in Greater China (Mainland China, Hong Kong, Macau, Taiwan).
The trial plans to include 175 patients and is part of the phase III NATiV3 study launched by Inventiva, a partner of Chia Tai Tianqing Pharmaceutical, which started in August 2021 and is expected to be completed in September 2025. Inventiva stated that if the NATiV3 study yields positive results, it can apply for the accelerated approval of Lanifibranor to the FDA, a milestone expected in the first half of 2026.
Previously, Inventiva had released the results of two Phase II clinical trials of lanifibranor. In the NATIVE study, which included 247 adult NASH patients with steatoclastis and moderate to severe necrotic inflammation and no cirrhosis, the proportion of patients in the lanifibranor 1200mg dose group who had at least a 2-point reduction in Steatosis-Activity-Fibrosis (SAF) activity score and no worsening of hepatic fibrosis was significantly higher than that in the placebo group (49% vs. 27%; P=0.004 or 55% vs. 34%; P=0.015).
In the IRB201800838-A study, which included 38 adult NASH patients with type 2 diabetes, liver fat content in the lanifibranor 800mg dose group fell significantly compared to the placebo group (-44% vs. -12%; P=0.002). In addition, the proportion of patients in the lanifibranor group who had reductions in liver fat content of more than 30% was significantly higher than that in the placebo group (65% vs. 22%; P=0.008).
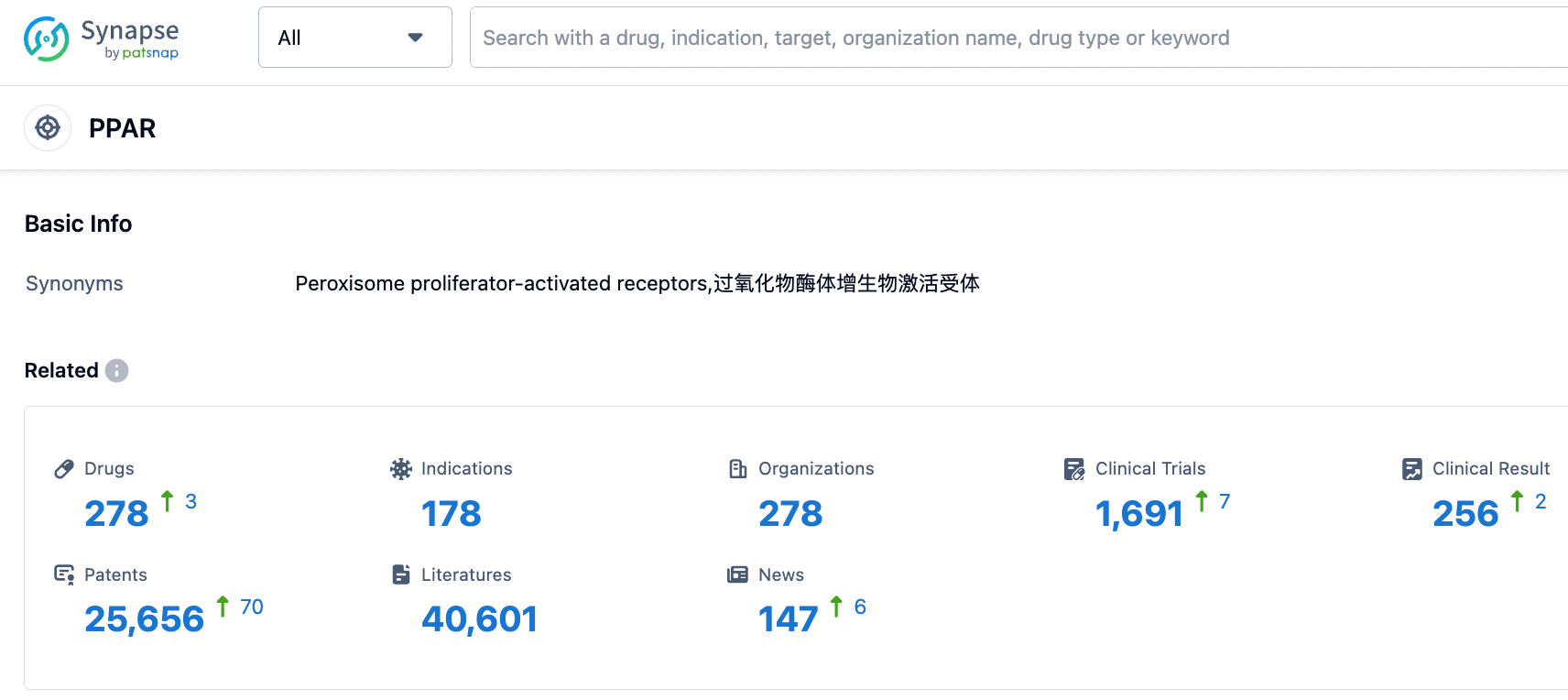
According to information disclosed by the Synapse database, as of September 12, 2023, there are a total of 278 drugs under development targeting PPAR, with 178 indications, 278 research institutions involved, 1690 related clinical trials, and as many as 25674 patents...Since NASH was discovered in 1980, the development of NASH drugs has faced many challenges, and the development of related targeted drugs has never been able to break through. The pipelines of leading pharmaceutical companies such as Gilead (simtuzumab and selonertib), Novartis (emricasan), and Pfizer (PF-06835919) have all ended in failure. The road ahead is unclear, and we look forward to the successful development of Lanifibranor, which may offer a new treatment option for NASH patients.