Cilnidipine: Detailed Review of its Transformative R&D Success
Cilnidipine's R&D Progress
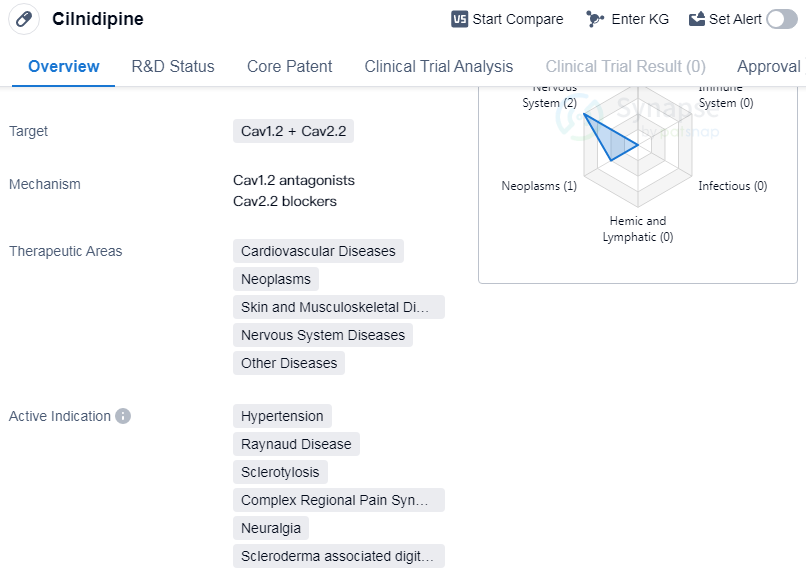
Cilnidipine is a small molecule drug that targets Cav1.2 and Cav2.2 channels. It has been approved for use in the treatment of various therapeutic areas, including cardiovascular diseases, neoplasms, skin and musculoskeletal diseases, nervous system diseases, and other diseases.
Cilnidipine was developed by Mochida Pharmaceutical Co., Ltd., a pharmaceutical organization based in Japan. It received its first approval in Japan in January 1995, making it available for use in the country. The drug has also obtained approvals in other countries, indicating its global recognition and acceptance.
As a small molecule drug, Cilnidipine works by targeting Cav1.2 and Cav2.2 channels. These channels are involved in various physiological processes, including the regulation of blood pressure and the transmission of pain signals. By modulating the activity of these channels, Cilnidipine can effectively manage hypertension and alleviate symptoms associated with conditions such as Raynaud disease, sclerotylosis, complex regional pain syndromes, neuralgia, and scleroderma associated digital ulcer.
The approval of Cilnidipine in multiple therapeutic areas highlights its versatility and potential for treating a wide range of diseases. Its efficacy in cardiovascular diseases, neoplasms, skin and musculoskeletal diseases, nervous system diseases, and other diseases suggests its potential to address various medical conditions. This makes Cilnidipine a valuable drug in the field of biomedicine.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Cilnidipine: Cav1.2 antagonists and Cav2.2 blockers
Cav1.2 antagonists and Cav2.2 blockers are terms related to biomedicine, specifically in the field of pharmacology.
Cav1.2 antagonists refer to drugs or compounds that inhibit or block the activity of the Cav1.2 calcium channel. Cav1.2 channels are voltage-gated calcium channels found in various tissues, including the heart and smooth muscle cells. By blocking the Cav1.2 channel, these antagonists can reduce the influx of calcium ions into cells, thereby affecting cellular processes and physiological functions regulated by calcium signaling. In the context of biomedicine, Cav1.2 antagonists may be used as therapeutic agents for conditions such as hypertension, cardiac arrhythmias, and certain neurological disorders.
On the other hand, Cav2.2 blockers are substances that specifically target and block the Cav2.2 calcium channel. Cav2.2 channels, also known as N-type calcium channels, are found primarily in the central nervous system and play a crucial role in neurotransmitter release. By blocking Cav2.2 channels, these blockers can modulate the release of neurotransmitters such as norepinephrine, glutamate, and substance P. This modulation can have therapeutic implications in the treatment of chronic pain, epilepsy, and other neurological disorders.
In summary, Cav1.2 antagonists and Cav2.2 blockers are types of drugs or compounds that target specific calcium channels in the body. They have potential applications in the treatment of various medical conditions, particularly those involving abnormal calcium signaling or neurotransmitter release.
Drug Target R&D Trends for Cilnidipine
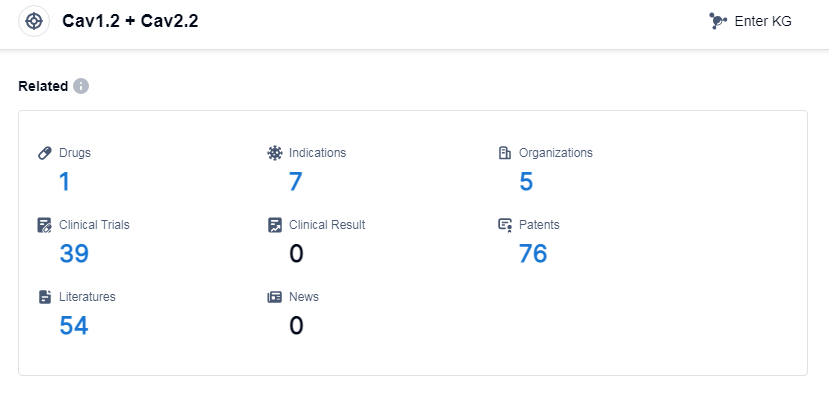
According to Patsnap Synapse, as of 13 Sep 2023, there are a total of 1 Cav1.2 and Cav2.2 drugs worldwide, from 5 organizations, covering 7 indications, and conducting 39 clinical trials.
The analysis of the current competitive landscape and future development of target Cav1.2 and Cav2.2 reveals several key findings. Mochida Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Bengbu Galaxy Biotech Co. Ltd. have emerged as leading companies in the development of drugs targeting this specific target, with successful approvals for their drugs. Hypertension is the most targeted indication, with one drug approved and another in an inactive phase. Small molecule drugs dominate the development of drugs targeting Cav1.2 and Cav2.2, indicating their effectiveness in this context. Japan, China, and the United States are the countries making the most progress in the development of drugs for this target, with China showing significant advancements. Overall, the analysis suggests a promising future for the development of drugs targeting Cav1.2 and Cav2.2, with potential applications in various indications and a strong presence in multiple countries.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Cilnidipine is a small molecule drug developed by Mochida Pharmaceutical Co., Ltd. It has been approved for use in Japan and other countries, demonstrating its global recognition. With its ability to target Cav1.2 and Cav2.2 channels, Cilnidipine offers therapeutic benefits in the treatment of hypertension, Raynaud Disease, Sclerotylosis, Complex Regional Pain Syndromes, Neuralgia, and Scleroderma associated digital ulcer. Its approval in multiple therapeutic areas further emphasizes its potential in addressing various diseases, making it a significant drug in the pharmaceutical industry.