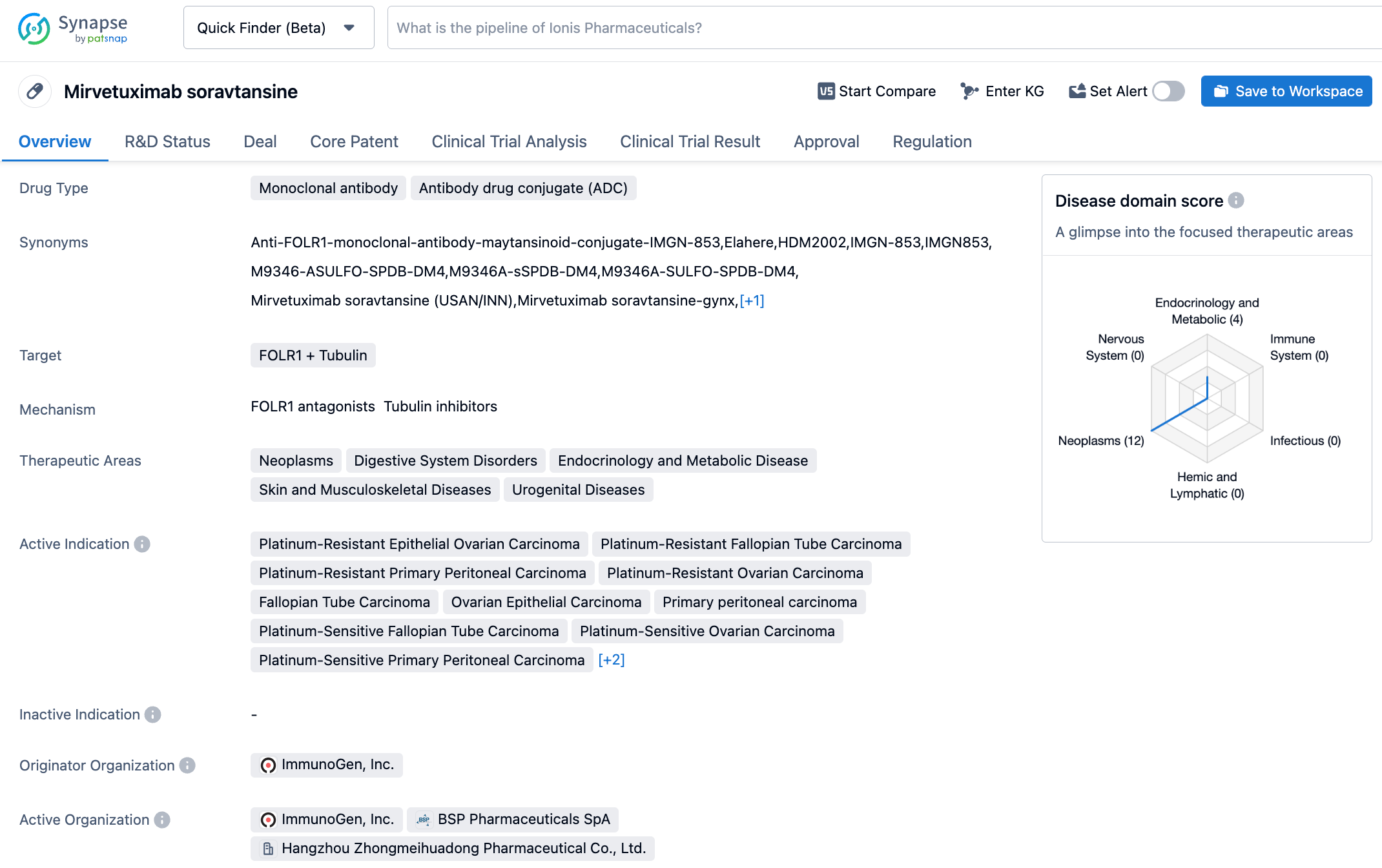
The marketing application for Huadong Medicine's FRα ADC Mirvetuximab Soravtansine Injection has been accepted
On October 25, 2023, Huadong Medicine announced that its wholly-owned subsidiary Hangzhou Zhongmeihuadong Pharmaceutical received a "Notice of Acceptance" issued by the China National Medical Products Administration (NMPA). The marketing authorization application for Mirvetuximab Soravtansine Injection (research code: IMGN853, HDM2002), jointly developed by Hangzhou Zhongmeihuadong and ImmunoGen, had been accepted. The submission is for the treatment of adults with epithelial ovarian, fallopian tube, or primary peritoneal cancer who are folate receptor alpha (FRα) positive and have platinum-resistant disease after 1-3 lines of systemic treatment.
Mirvetuximab soravtansine is the world's first ADC drug for FRα positive ovarian cancer developed by Huadong Medicine in cooperation with ImmunoGen. The product received accelerated approval from the U.S. FDA in November 2022 for the treatment of adults with epithelial ovarian, fallopian tube, or primary peritoneal cancer that is FRα positive and have been treated with 1-3 lines of systemic therapy. In October 2020, Huadong Medicine reached an agreement with ImmunoGen, obtaining the development and commercialization rights for Mirvetuximab soravtansine in China for an upfront payment of $40 million, up to $265 million in milestone payments, and a predetermined percentage of the sales commission. ImmunoGen retains all rights outside of China.
The study enrolled 106 patients with platinum-resistant advanced serous epithelial ovarian, primary peritoneal or fallopian tube cancers. The objective response rate (ORR) assessed by the investigators was 32.4%, and the ORR assessed by BICR was 31.6%. According to ImmunoGen's financial report, Mirvetuximab Soravtansine is gradually stepping up its dosage, with sales revenue reaching $29.5 million in Q1 2023. ImmunoGen is also conducting a confirmatory phase III MIRASOL study aimed at transitioning Mirvetuximab Soravtansine's accelerated approval in the U.S. to full approval. In May 2023, the MIRASOL study reported that compared to chemotherapy, Mirvetuximab Soravtansine extended patients' overall survival (OS) from 12.75 months to 16.46 months, reducing their risk of death by 33%, thus becoming the first new drug to extend the OS of patients with FRα positive platinum-resistant ovarian cancer. The progression-free survival (PFS) was extended from 3.98 months to 5.62 months.
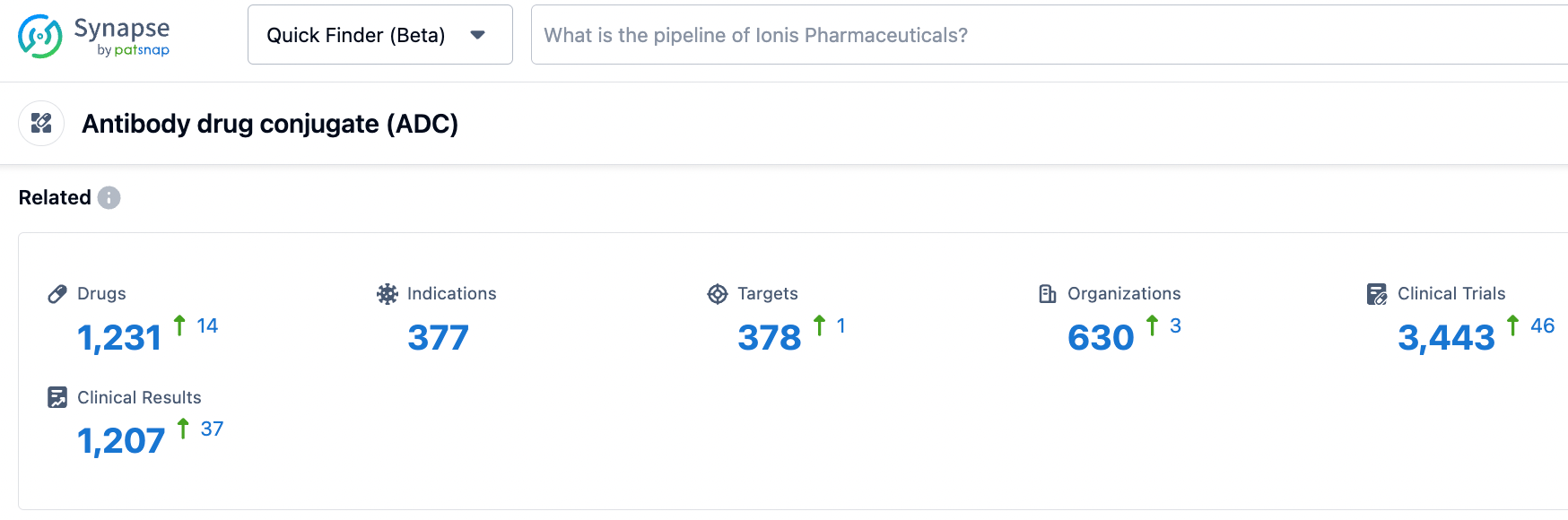
According to information disclosed by the synapse database, as of October 26, 2023, there were 1231 ADC drugs in development, involving 377 indications, 378 targets, 630 research institutes, and 3444 clinical trials. We are hoping for the early launch of Mirvetuximab Soravtansine to bring new treatment options for patients.