CinFina Pharma Gains US Approval for Early Obesity Drug Trial, Begins Dosing
CinFina Pharma, an enterprise operating under the CinRx group specializing in the development of effective therapeutic solutions targeting obesity and metabolic disorders, has recently communicated that the U.S. Food and Drug Administration has granted authorization for their Investigational New Drug (IND) Application concerning CIN-110, which is a synthetic analog of aPYY3-36. This approval paves the way for the pioneering clinical trials involving human subjects to begin. At the onset of this clinical investigation, CinFina has also shared the news that the initial group of enrolled subjects has received their first dosage.
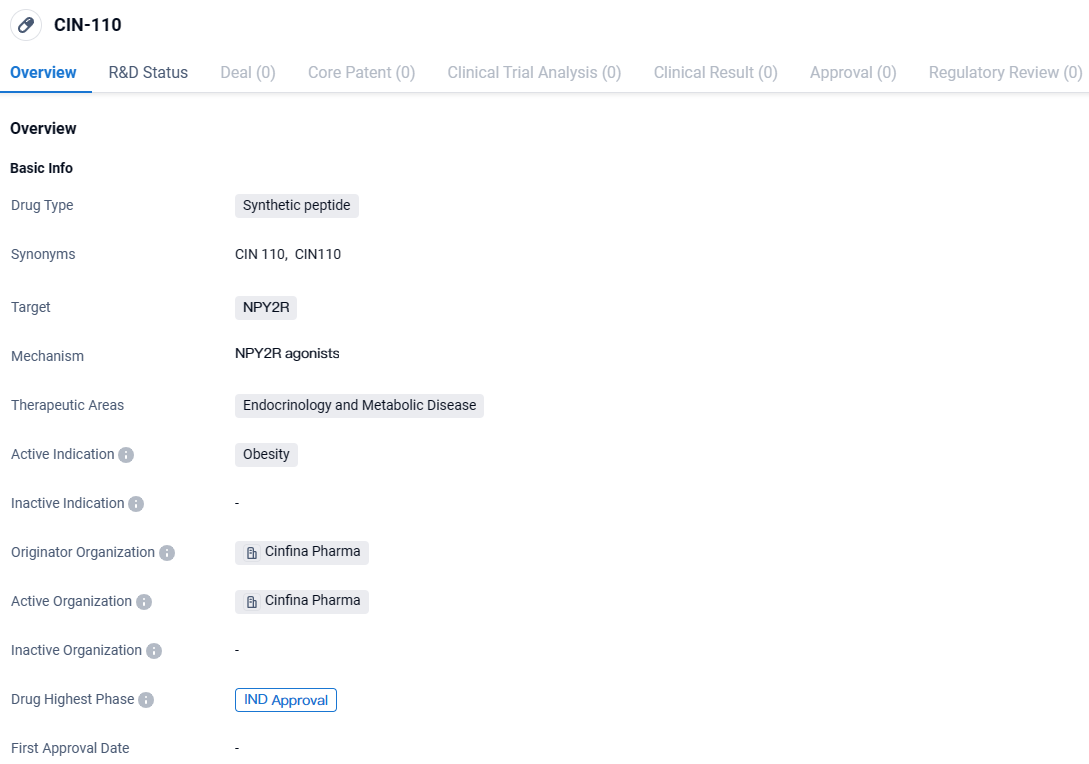
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
 The forthcoming clinical assessment will examine the effects of CIN-110 with respect to safety profiles, dose tolerance, drug absorption/distribution/metabolism/excretion characteristics, functional effects on biological pathways, and potential to provoke immune responses. This study will employ a systematic, blinded, and placebo-managed methodology, escalating the dose for each participant, focusing on individuals who are otherwise healthy but classified as obese.
The forthcoming clinical assessment will examine the effects of CIN-110 with respect to safety profiles, dose tolerance, drug absorption/distribution/metabolism/excretion characteristics, functional effects on biological pathways, and potential to provoke immune responses. This study will employ a systematic, blinded, and placebo-managed methodology, escalating the dose for each participant, focusing on individuals who are otherwise healthy but classified as obese.
"There is an ongoing necessity for effective medications capable of being used safely over prolonged periods when combating the growing obesity crisis," remarked Dr. Jon Isaacsohn, the pioneering executive at CinRx Pharma. "CIN-110, inspired by its favorable results in initial laboratory tests, stands poised to fulfill these therapeutic gaps, offering patients significant advantages when used alone or in drug combinations, including sustained reductions in body weight."
Developed as a treatment both alone and for use alongside other medications for obesity, CIN-110 is a synthesized variant of the naturally occurring hormone PYY3-36. PYY3-36 is typically released by the intestinal system and plays a key role in hunger suppression and the regulation of caloric intake by engaging the Y2 receptor in the brain. CIN-110 stands out due to its engineered structure aimed to minimize the intense nausea and vomiting associated with earlier PYY-related treatments while maintaining its efficacy for long-lasting weight management.
CIN-110 is distinctly tailored to interact with and stimulate Y2R, a receptor that normally binds with PYY3-36 within the body. A comprehensive examination of CIN-110's pharmacodynamics reveals that its continued skin injections notably lessen food consumption and decrease body mass in overweight test subjects such as non-human primates and various rodent species. Research also indicates that CIN-110 contributed to enhanced glucose balance and better insulin responsiveness in these animal models.
The findings from the toxicological analysis suggest that CIN-110 exhibits a favorable tolerance profile, not eliciting harmful effects in non-human primates or rodent subjects. Evidence from these initial effectiveness and safety evaluations has paved the way for CIN-110 to advance into clinical investigations involving human participants.
"CIN-110's laboratory development emphasizes the innovative potential of PYY in treating obesity with a method that sets it apart from other therapeutics," stated Dr. Brian Murphy from CinRx Pharma. "With its distinctive molecular design, the molecule promises a new dimension to current treatments, with improved specificity, effectiveness, and an extended duration of action beyond that of GLP-1 analogs."
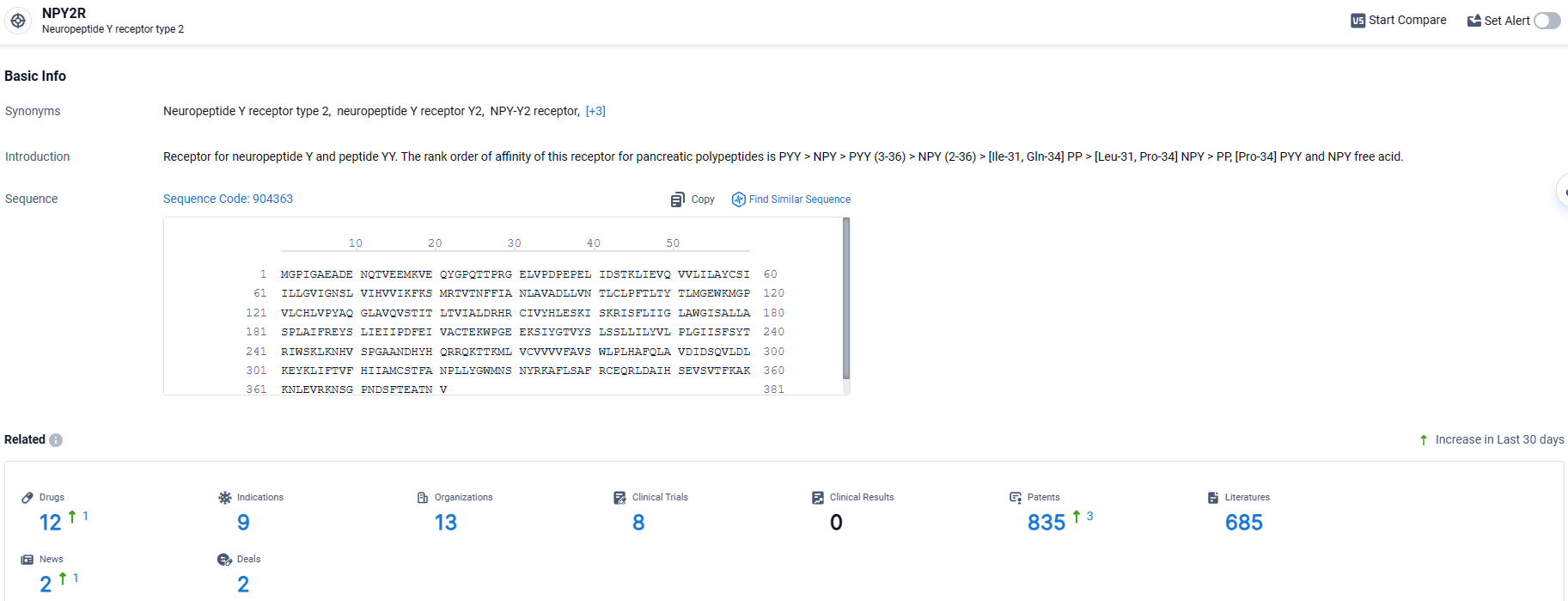
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of March 28 2024, there are 12 investigational drugs for the NPY2R target, including 9 indications, 13 R&D institutions involved, with related clinical trials reaching 8, and as many as 835 patents.
CIN-110, it is engineered to interact with the NPY2R receptor. Obesity, an active indication for CIN-110, is a complex disease characterized by excessive body fat accumulation. It is a major public health problem worldwide, leading to various health problems such as cardiovascular disease, diabetes, and certain types of cancer.