Clindamycin Phosphate Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs, Mechanisms of Action, and Drug Target
Clindamycin Phosphate's R&D Progress
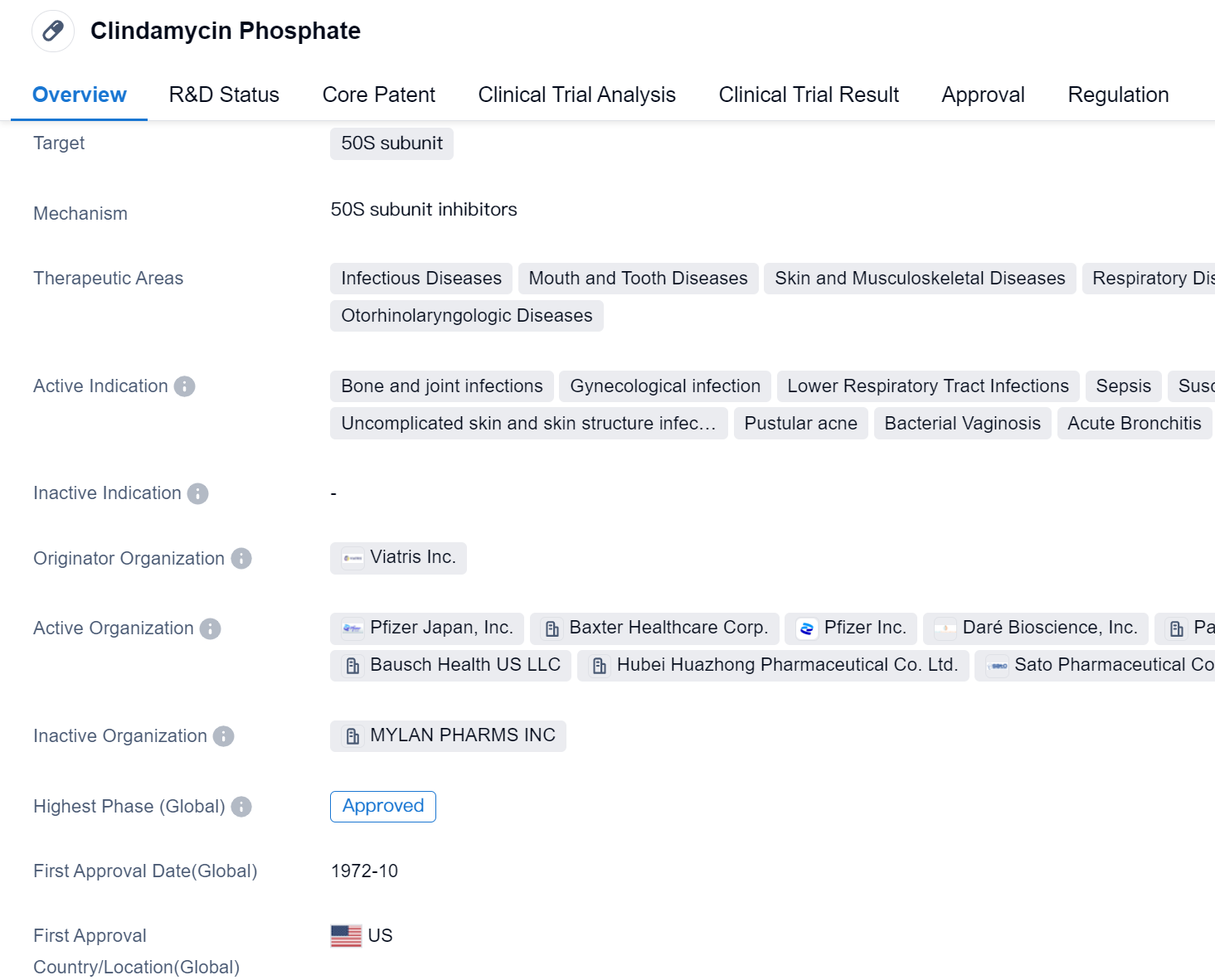
Clindamycin Phosphate is a small molecule drug that targets the 50S subunit. It is primarily used in the treatment of various infectious diseases, including bone and joint infections, gynecological infections, lower respiratory tract infections, sepsis, and susceptible anaerobic bacteria infections. It is also effective in treating uncomplicated skin and skin structure infections, pustular acne, bacterial vaginosis, acute bronchitis, hemorrhagic septicemia, laryngitis, otitis media, pharyngitis, pneumonia, secondary infections, sinusitis, tonsillitis, acne vulgaris, bacterial infections, complicated skin and soft tissue infections, intraabdominal infections, reproductive tract infections, and respiratory tract infections.
The drug was first approved in the United States in October 1972 and has since gained approval in other countries. It is classified as a fast-track drug, indicating that it has undergone expedited regulatory processes due to its potential to address unmet medical needs.
Clindamycin Phosphate is developed by Viatris Inc., an originator organization in the pharmaceutical industry. The drug has reached the highest phase of development which is approved globally. This signifies that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
As a small molecule drug, Clindamycin Phosphate is designed to interact with the 50S subunit, a component of bacterial ribosomes. By targeting this subunit, the drug inhibits protein synthesis in bacteria, leading to their eventual death or growth inhibition. This mechanism of action makes Clindamycin Phosphate effective against a wide range of bacterial infections.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Clindamycin Phosphate: 50S subunit inhibitors
From a biomedical perspective, 50S subunit inhibitors are a type of drugs that target the 50S ribosomal subunit in bacteria. The ribosome is a cellular structure responsible for protein synthesis. In bacteria, 50S subunit is a component of the bacterial ribosome, and inhibiting its function can disrupt bacterial protein synthesis, leading to a halt in bacterial growth and replication.
These inhibitors work by binding to the 50S subunit and interfering with its ability to catalyze the formation of peptide bonds during protein synthesis. By blocking this essential step, the inhibitors prevent the synthesis of new proteins necessary for bacterial survival.
Examples of 50S subunit inhibitors include macrolide antibiotics such as erythromycin, clarithromycin, and azithromycin. These drugs are commonly used to treat various bacterial infections, including respiratory tract infections, skin infections, and certain sexually transmitted diseases.
It is important to note that 50S subunit inhibitors specifically target bacterial ribosomes and are not effective against human ribosomes. This selectivity allows these drugs to selectively inhibit bacterial protein synthesis without affecting human cells, minimizing potential side effects.
Drug Target R&D Trends for Clindamycin Phosphate
50S subunit is a crucial component of the ribosome, which is responsible for protein synthesis in the human body. It plays a vital role in the translation of genetic information from mRNA into functional proteins. The 50S subunit, along with the 30S subunit, forms the complete ribosome structure. It provides the catalytic site for peptide bond formation during protein synthesis. Additionally, the 50S subunit is involved in the binding of tRNA molecules and the translocation of the ribosome along the mRNA strand. Overall, the 50S subunit is essential for the accurate and efficient production of proteins, which are essential for various biological processes in the human body.
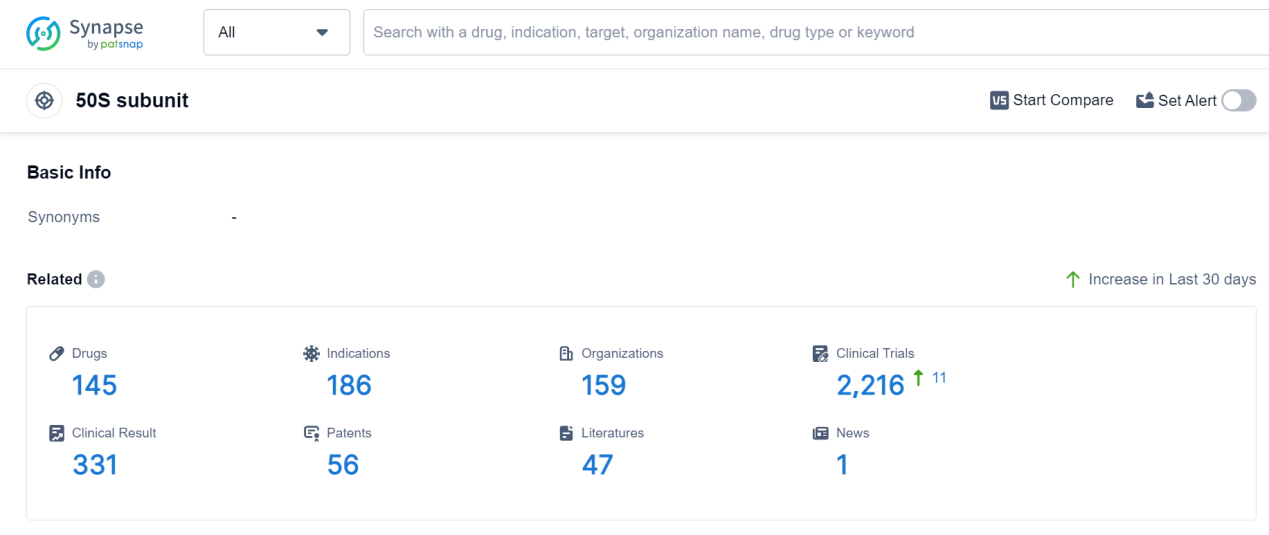
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 145 50S subunit drugs worldwide, from 159 organizations, covering 186 indications, and conducting 2216 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Clindamycin Phosphate is a small molecule drug developed by Viatris Inc. It targets the 50S subunit and is approved for the treatment of various infectious diseases. Its broad therapeutic areas include infectious diseases, mouth and tooth diseases, skin and musculoskeletal diseases, respiratory diseases, urogenital diseases, and otorhinolaryngologic diseases. With its long history of use since 1972 and fast-track regulatory status, Clindamycin Phosphate has established itself as a reliable and effective treatment option in the pharmaceutical industry.