Pharmaceutical Insights: Ceftazidime's R&D Progress and its Mechanism of Action on Drug Target
Ceftazidime's R&D Progress
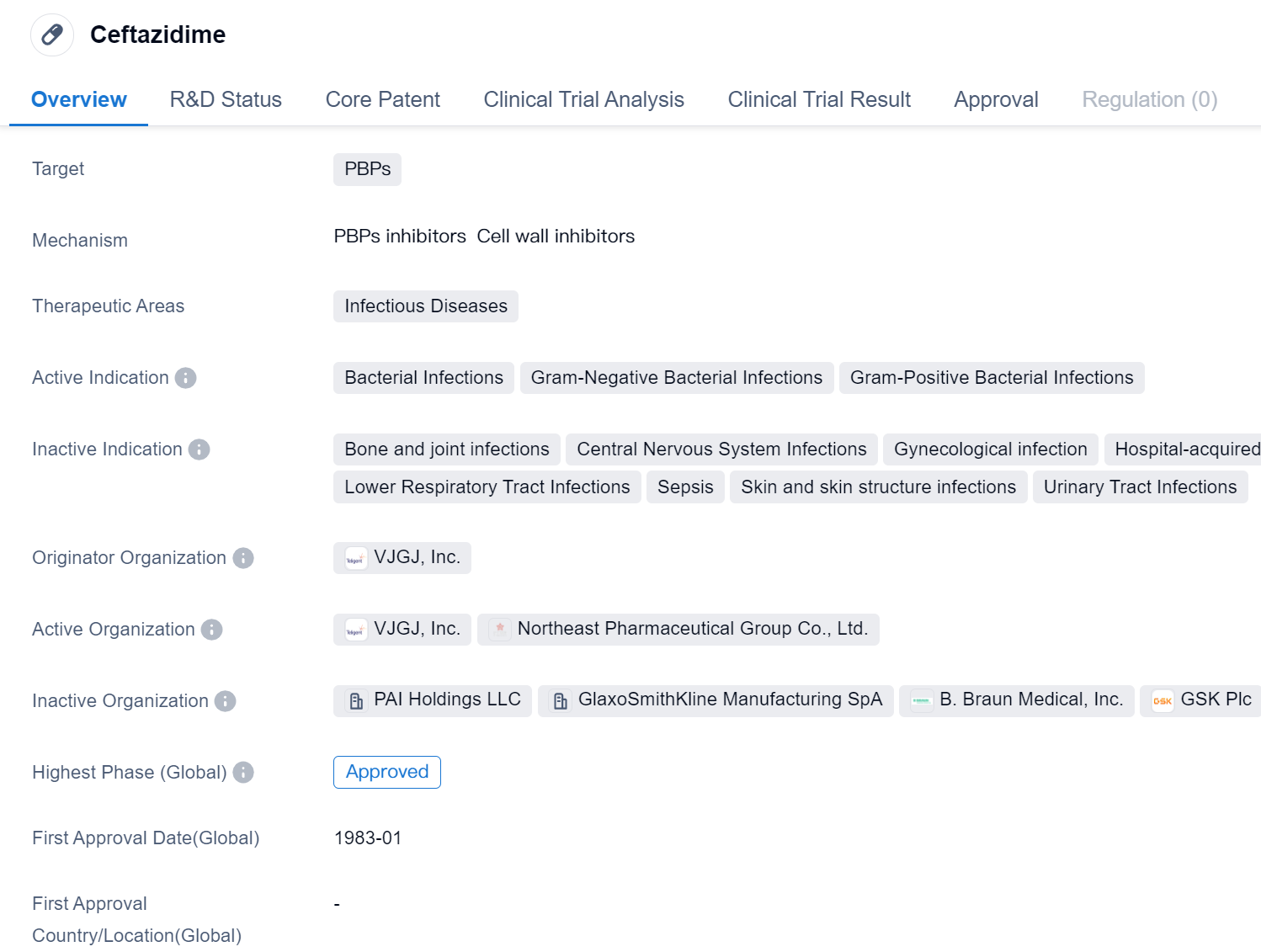
Ceftazidime is a small molecule drug that falls under the category of biomedicine. It primarily targets PBPs (penicillin-binding proteins) and is used in the treatment of infectious diseases. The drug has been approved for use in both global and Chinese markets.
Ceftazidime is indicated for the treatment of bacterial infections, specifically gram-negative and gram-positive bacterial infections. Gram-negative bacteria are a type of bacteria that have a thin peptidoglycan layer in their cell wall, making them more resistant to antibiotics. Gram-positive bacteria, on the other hand, have a thick peptidoglycan layer in their cell wall, making them more susceptible to antibiotics.
The drug was first approved globally in January 1983, indicating its long-standing presence in the pharmaceutical market. It was developed by VJGJ, Inc., an originator organization that played a crucial role in bringing this drug to market.
As a small molecule drug, ceftazidime is designed to interact with specific targets in the body, in this case, PBPs. PBPs are enzymes involved in the synthesis of bacterial cell walls. By targeting these proteins, ceftazidime disrupts the formation of the bacterial cell wall, leading to the death of the bacteria.
Infectious diseases pose a significant threat to public health, and the approval of ceftazidime provides healthcare professionals with an effective tool to combat bacterial infections. The drug's approval in global markets highlights its widespread recognition and acceptance in the medical community.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ceftazidime: PBPs inhibitors Cell wall inhibitors
PBPs inhibitors are a type of medication that target and inhibit the activity of penicillin-binding proteins (PBPs). PBPs are enzymes found in the cell wall of bacteria, specifically in the peptidoglycan layer. They play a crucial role in the synthesis and maintenance of the bacterial cell wall.
By inhibiting PBPs, these inhibitors disrupt the formation of the bacterial cell wall, leading to cell wall instability and ultimately bacterial cell death. This mechanism of action makes PBPs inhibitors effective against a wide range of bacterial infections.
Cell wall inhibitors, on the other hand, are a broader category of drugs that target various components involved in the synthesis or integrity of the bacterial cell wall. PBPs inhibitors are a specific subset of cell wall inhibitors that specifically target the PBPs.
These medications are commonly used in the treatment of bacterial infections, such as pneumonia, skin infections, urinary tract infections, and certain types of meningitis. They are often used in combination with other antibiotics to enhance their effectiveness and prevent the development of antibiotic resistance.
It is important to note that PBPs inhibitors and other cell wall inhibitors are specific to bacteria and do not have any effect on human cells. This specificity allows for targeted treatment of bacterial infections while minimizing potential side effects on the patient.
Drug Target R&D Trends for Ceftazidime
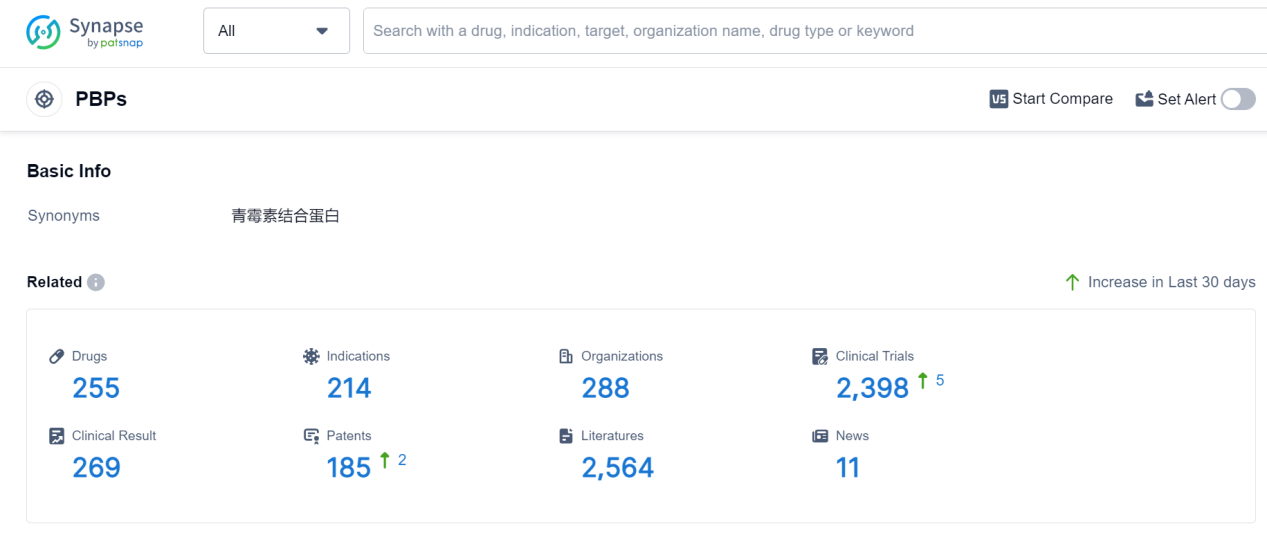
PBPs, or Penicillin-Binding Proteins, play a crucial role in the human body. These proteins are found in the cell walls of bacteria and are responsible for the synthesis and maintenance of the peptidoglycan layer, a vital component of bacterial cell walls. PBPs act as targets for antibiotics, particularly beta-lactam antibiotics like penicillin, by binding to them and inhibiting the enzymes that cross-link the peptidoglycan strands. This disruption weakens the bacterial cell wall, leading to cell lysis and ultimately bacterial death. Understanding the role of PBPs is essential in developing effective antibiotics and combating bacterial infections.
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 255 PBPs drugs worldwide, from 288 organizations, covering 214 indications, and conducting 2398 clinical trials.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, ceftazidime is a small molecule drug developed by VJGJ, Inc. that targets PBPs and is used in the treatment of infectious diseases, particularly gram-negative and gram-positive bacterial infections. Its approval in 1983 signifies its long-standing presence in the pharmaceutical market, and its effectiveness in combating bacterial infections makes it a valuable asset in the field of biomedicine.