The Research and Development Boom is Unstoppable-KRAS G12D Inhibitors
The KRAS gene is one of the most commonly mutated oncogenes in tumors and can be detected in various types of malignant tumors, being particularly prevalent in pancreatic cancer (86%), colorectal cancer (41%), and lung cancer (32%). Among all KRAS mutations, G12D (35%), G12V (29%), and G12C (21%) are the most common.
KRAS G12D differs from KRAS G12C only in the type of amino acid mutation on the same codon. KRAS G12D is a mutation that introduces an aspartic acid instead of a cysteine at the 12th codon of KRAS, resulting in epidemiological differences.
KRAS G12C is the most common KRAS mutation in NSCLC patients, while KRAS G12D is the most common mutation in colorectal cancer and pancreatic cancer. KRAS mutations are independent prognostic factors for colorectal cancer patients.
Pancreatic cancer, known as the "king of cancers", has extremely limited treatment options and drugs, and the 5-year survival rate of patients is only 10%. Most patients only live about one year after diagnosis, the lowest survival rate of all common cancers. Research shows that about 90% of pancreatic cancer patients have KRAS mutations, with the KRAS G12D mutation accounting for the highest proportion at 45% (G12C mutations only account for 1%).
There is substantial evidence that, compared to other KRAS mutations, the G12D mutation confers a stronger immunosuppressive tumor microenvironment and higher resistance to immunotherapy. In the subcutaneous injection model in mice, anti-PD1 monotherapy was used to treat tumors from genetically identical mice with different G12 mutations of non-small cell lung cancer (NSCLC). Compared to other KRAS mutations, tumors with the G12D mutation displayed increased resistance to anti-PD1 treatment, decreased expression of Programmed Death-Ligand 1 (PD-L1) on tumor cells, and reduced infiltration of cytotoxic CD8+ T cells into the tumor. Similar observations have been made in NSCLC patients: compared to tumors with the G12C mutation, KRAS-G12D tumors show reduced PD-L1 expression and CD8+ T cell infiltration.
KRAS G12D is a specific mutation of the KRAS gene that plays a crucial role in the development and progression of various cancers. This mutation occurs in the G12D codon of the KRAS gene, leading to a change in the amino acid sequence of the KRAS protein. The KRAS G12D mutation is commonly found in pancreatic, colorectal, and lung cancers, among others. It results in the constitutive activation of the KRAS protein, leading to uncontrolled cell growth, proliferation, and survival. Understanding the role of KRAS G12D is essential for developing targeted therapies that can effectively inhibit its activity and provide potential treatment options for patients with KRAS-mutated cancers.
KRAS G12D Competitive Landscape
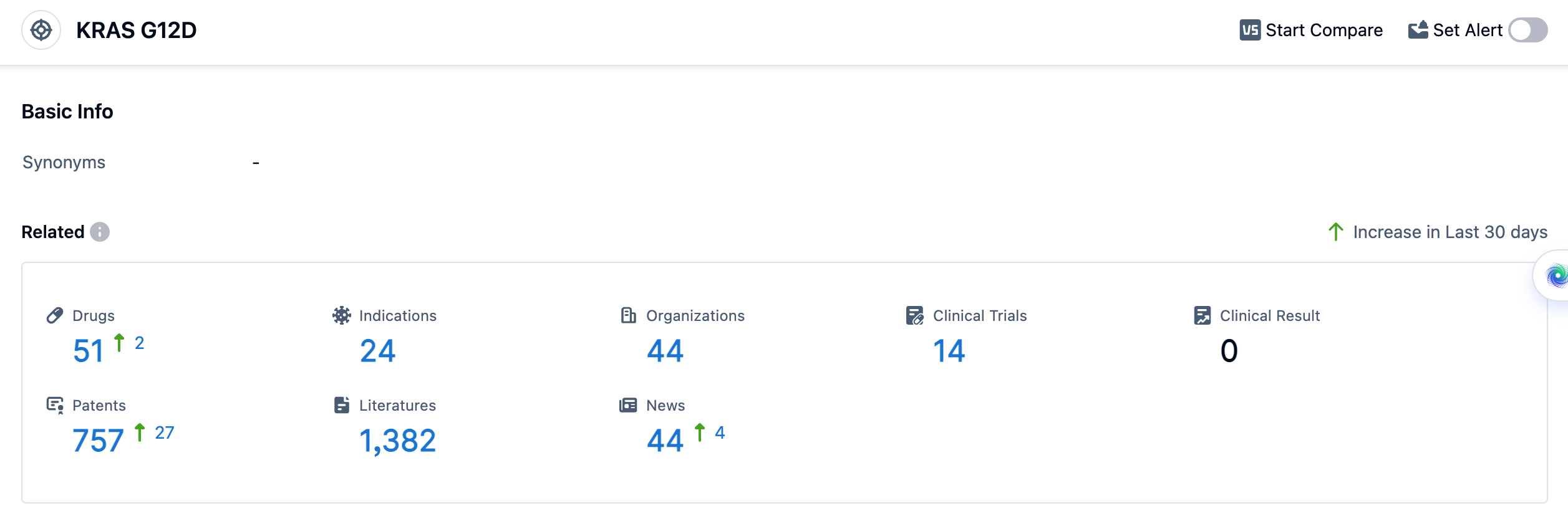
According to the data provided by Patsnap Synapse-Global Drug Intelligence Database: the following figure shows that as of 11 Sep 2023, there are a total of 51 KRAS G12D drugs worldwide, from 44 organizations, covering 24 indications, and conducting 14 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The approved indications for drugs targeting KRAS G12D include pancreatic cancer, colorectal cancer, non-small cell lung cancer, and various solid tumors. These indications are being targeted in different phases of development, including Phase 1/2, Phase 1, and preclinical stages. The high number of drugs in preclinical development suggests a strong focus on exploring the potential of KRAS G12D in various cancer types.
The analysis of the target KRAS G12D reveals a competitive landscape with multiple companies actively involved in R&D. Silenseed Ltd., Mirati Therapeutics, Inc., and C.H. Boehringer Sohn AG & Co. KG are among the companies growing fastest under this target. The approved indications for drugs targeting KRAS G12D include pancreatic cancer, colorectal cancer, non-small cell lung cancer, and various solid tumors. Small molecule drugs and siRNA are progressing rapidly, indicating intense competition and a focus on innovative drug development. The United States, Israel, and China are leading in terms of drug development, with China showing significant progress. Overall, the future development of target KRAS G12D holds promise for advancing the treatment options for various cancer types.
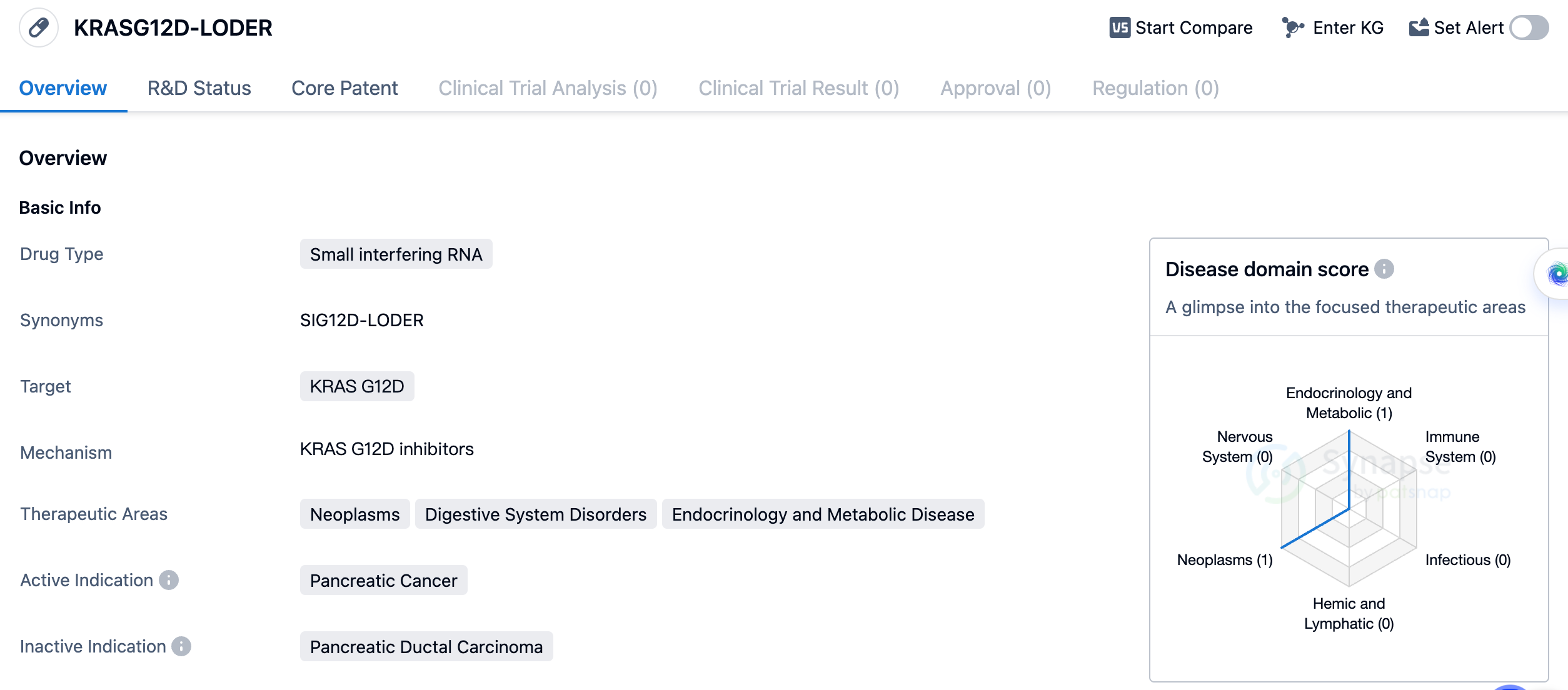
Researching siRNA Therapy:KRASG12D-LODER
The drug KRASG12D-LODER is a small interfering RNA (siRNA) that targets the KRAS G12D gene. It is being developed for the treatment of pancreatic cancer, a type of neoplasm that affects the digestive system. Additionally, it has potential therapeutic applications in the fields of endocrinology and metabolic diseases. The drug is being developed by Silenseed Ltd., an organization specializing in biomedicine. As of now, KRASG12D-LODER has reached the highest phase of clinical development, which is Phase 2.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
siRNAs are a class of molecules that can selectively silence or inhibit the expression of specific genes. In the case of KRASG12D-LODER, it specifically targets the KRAS G12D gene. KRAS mutations, including the G12D mutation, are commonly found in pancreatic cancer and are associated with tumor growth and resistance to treatment. By targeting this specific mutation, KRASG12D-LODER aims to inhibit the growth and spread of pancreatic cancer cells.
Pancreatic cancer is a highly aggressive and difficult-to-treat cancer with limited treatment options. Therefore, the development of targeted therapies like KRASG12D-LODER holds great promise in improving patient outcomes. If successful, this drug could potentially provide a more effective and personalized treatment option for patients with pancreatic cancer.
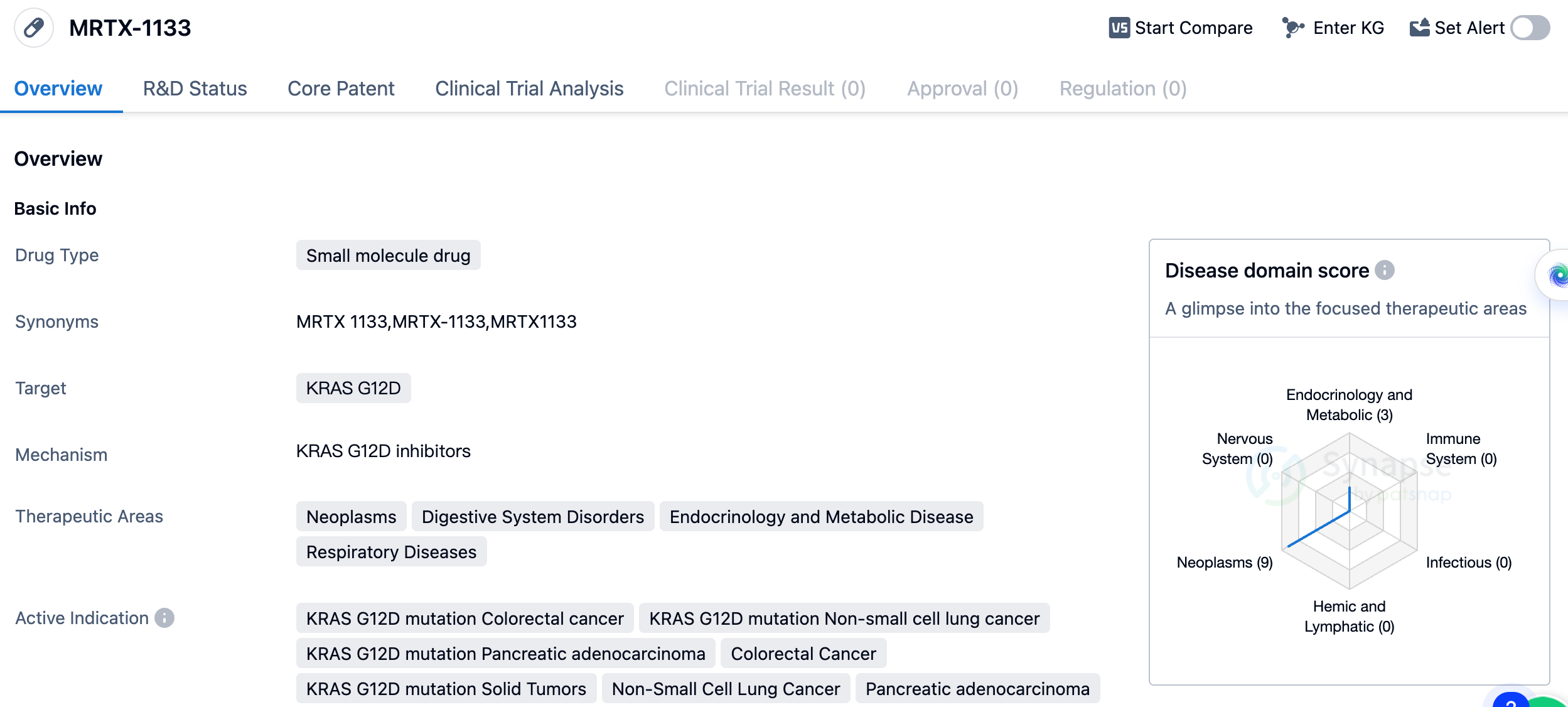
MRTX1133
MRTX-1133 is a small molecule drug developed by Mirati Therapeutics, Inc. It falls under the category of biomedicine and specifically targets the KRAS G12D mutation. This mutation is associated with various types of cancers, including colorectal cancer, non-small cell lung cancer, and pancreatic adenocarcinoma. The therapeutic areas in which MRTX-1133 is expected to be effective include neoplasms (abnormal growth of cells), digestive system disorders, endocrinology and metabolic diseases, and respiratory diseases. This suggests that the drug has the potential to treat a wide range of conditions related to these areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The active indications for MRTX-1133 include colorectal cancer with the KRAS G12D mutation, non-small cell lung cancer with the KRAS G12D mutation, pancreatic adenocarcinoma with the KRAS G12D mutation, colorectal cancer, solid tumors with the KRAS G12D mutation, non-small cell lung cancer, pancreatic adenocarcinoma, neoplasms, and pancreatic cancer. These indications highlight the drug's potential to target specific mutations and various types of cancer.
In summary, MRTX-1133 is a small molecule drug developed by Mirati Therapeutics, Inc. It targets the KRAS G12D mutation and shows potential in treating neoplasms, digestive system disorders, endocrinology and metabolic diseases, and respiratory diseases. The drug is currently in Phase 1/2 of development and has active indications for various types of cancer, including colorectal cancer, non-small cell lung cancer, and pancreatic adenocarcinoma.