Clinical Trial Outcomes Indicate Similar Efficacy Between Alvotech's Biosimilar AVT06 and Its Comparator, Eylea® (Aflibercept)
Alvotech, an international biopharmaceutical enterprise dedicated to creating and producing biosimilar treatments aimed at a global patient demographic, has disclosed encouraging preliminary findings from a pivotal clinical trial concerning AVT06, which is Alvotech's potential biosimilar counterpart to Eylea® (aflibercept).
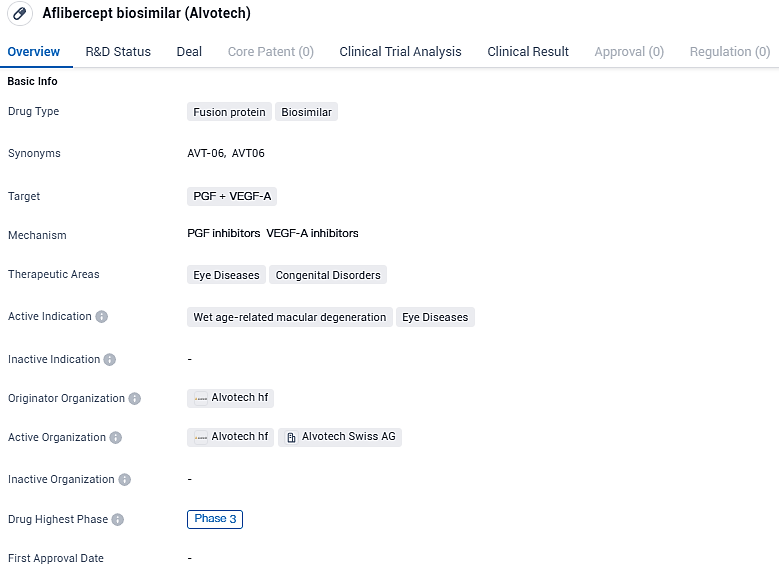
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Alvotech's Chairman and CEO, Robert Wessman, noted the advancement within their product range, highlighting the efficiency of their comprehensive production and developmental infrastructure. This structure has empowered Alvotech to actively engage in its varied worldwide pursuit of biosimilars. Wessman underscored the importance of reaching a significant clinical phase for AVT06, which underlines the company's capability to swiftly progress an array of high-quality biosimilar candidates in tandem.
Regeneron's Eylea is well-established in the pharmaceutical market as a biologic agent for a range of ocular conditions that pose a threat to vision, potentially leading to significant visual impairment or total vision loss. Notable conditions treated by Eylea include wet age-related macular degeneration (AMD), macular edema following retinal vein occlusion, and diabetic eye disease. Eylea's worldwide sales reached approximately $9.4 billion in the one-year period leading up to September 30, 2023.
Alvotech conducted the pivotal study labeled AVT06-GL-C01, a substantial trial designed to methodically assess the efficacy, safety profile, and immunogenic response of AVT06 in relation to Eylea in patients affected by neovascular AMD. This rigorous, controlled trial involved a randomized, double-masked method, engaging multiple centers, and conducted parallel to established groups.
The core metric of success in the study was the change noted at the 8-week mark from the baseline in Best-Corrected Visual Acuity (BCVA). The findings were affirmative; the trial achieved its primary aim, successfully establishing that there were no significant differences in the therapeutic impact when comparing AVT06, Alvotech's biosimilar, with the reference biologic, Eylea.
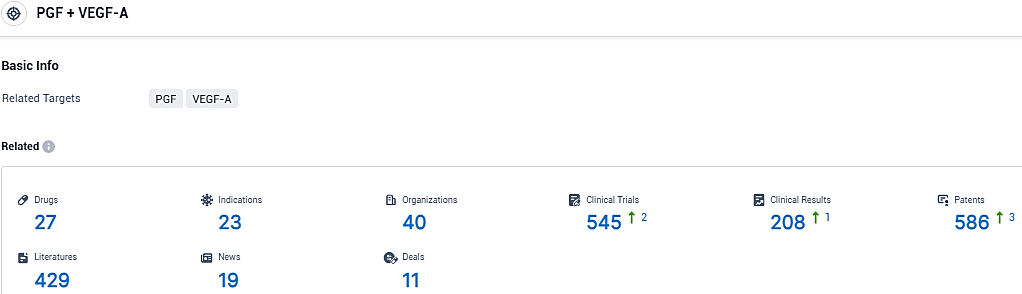
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of January 6, 2024, there are 27 investigational drugs for the PGF and VEGF-A target, including 23 indications, 40 R&D institutions involved, with related clinical trials reaching 208, and as many as 586 patents.
AVT06 is a recombinant fusion protein and a biosimilar candidate to Eylea® (aflibercept), which binds vascular endothelial growth factors (VEGF), inhibiting the binding and activation of VEGF receptors, neovascularization, and vascular permeability. AVT06 is an investigational product and has not received regulatory approval in any country. Biosimilarity has not been established by regulatory authorities and is not claimed.