Coherus and Junshi Biosciences reveal FDA validation for LOQTORZI™ (toripalimab-tpzi) in all treatment phases for repeated or spreading Nasopharyngeal Cancer (NPC)
Coherus BioSciences, Inc. along with Shanghai Junshi Biosciences Co., Ltd. revealed that the FDA has given its approval for LOQTORZI™ (toripalimab-tpzi) to be used with cisplatin and gemcitabine. This combination has been approved as the initial therapy for adult patients diagnosed with metastatic or recurrent advanced NPC. Additionally, LOQTORZI™ (toripalimab-tpzi) has also been approved as a standalone treatment for adult patients suffering from recurrent, inoperable, or metastatic NPC, especially for those showing disease progression subsequent to undergoing platinum-based chemotherapy.
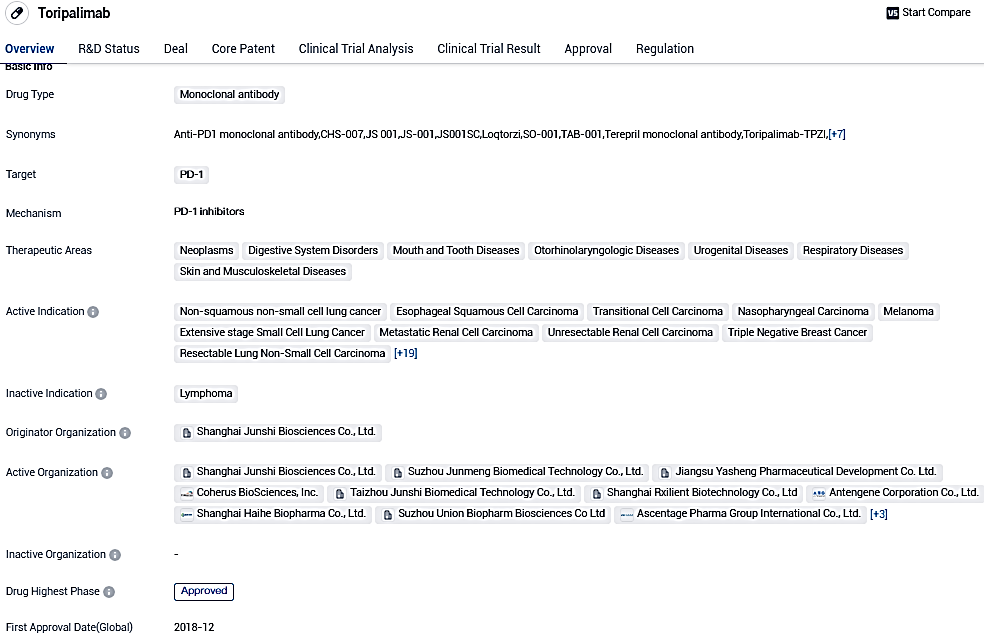
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Based on the evidence from JUPITER-02 Phase 3 and POLARIS-02 Phase 2 research, the authorization was given independent of a patient’s PD-L1 status. LOQTORZI stands as a novel PD-1 monoclonal antibody, efficiently obstructing PD-1 ligands PD-L1 and PD-L2 at a distinctive site on the PD-1 receptor and enhancing the immune system’s ability to fight and eradicate tumors.
In the JUPITER-02 Phase 3 trial, LOQTORZI in combination with chemotherapy substantially advanced progression-free survival, lowering the chance of disease advancement or death by 48% compared to just chemotherapy. LOQTORZI also showed an important and clinically impactful improvement in overall survival, leading to a 37% decrease in mortality risk compared to chemotherapy alone.
NPC is a violent cancer that originates from the nasopharynx – the upper section of the throat behind the nose by the skull base. Due to the tumour's location, surgery usually isn't an option and patients with localized disease are mainly treated with radiation and chemotherapy. LOQTORZI is the first FDA authorized drug for NPC patients.
“This initial approval of LOQTORZI marks a milestone for Coherus as a trailblazer in cancer therapy," commented Coherus Chairman and CEO Denny Lanfear. "As a novel PD-1 inhibitor, it's proving vital to our I-O approach to prolonging cancer patient survival as demonstrated by the remarkable NPC results."
Lanfear added, "We're particularly enthusiastic about focusing on broadening the use of LOQTORZI across multiple tumor types in combination with I-O agents that target the tumor microenvironment. This includes our IL27-targeted antibody casdozokitug, and our CCR8 inhibitor CHS-114, potentially massively increasing the number of cancer patients experiencing enhanced survival."
“The FDA’s endorsement of LOQTORZI for NPC patients who currently have very restricted treatment options is particularly assuring, presenting new therapies to manage this violent and potentially fatal form of cancer,” mentioned Jong Chul Park, M.D., Assistant Professor, Harvard Medical School and attending physician at the Center for Head and Neck Cancers at Massachusetts General Hospital Cancer Center.
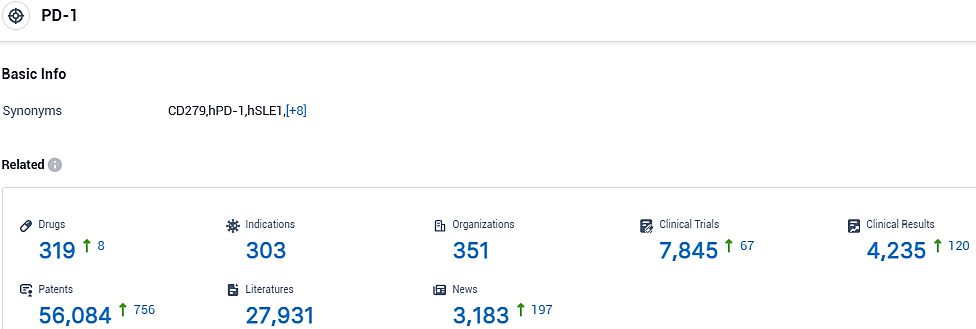
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 1, 2023, there are 319 investigational drugs for the PD-1 target, including 303 indications, 351 R&D institutions involved, with related clinical trials reaching 7845, and as many as 56084 patents.
Toripalimab embodies a remarkable progress in biomedicine, prominently in the management of diverse cancers. Its endorsements across numerous medical fields and its regulatory statuses showcase its capability to be a beneficial therapeutic choice. The medication's favorable outcomes in clinical investigations, along with approval within China and at an international scale, signify its effectiveness and safety. As research and usage of toripalimab persist, it holds promise in substantially influencing the wellbeing of patients with various conditions.