Lotilaner Unveiled: A Detailed Overview of its Revolutionary R&D Breakthroughs
Lotilaner's R&D Progress
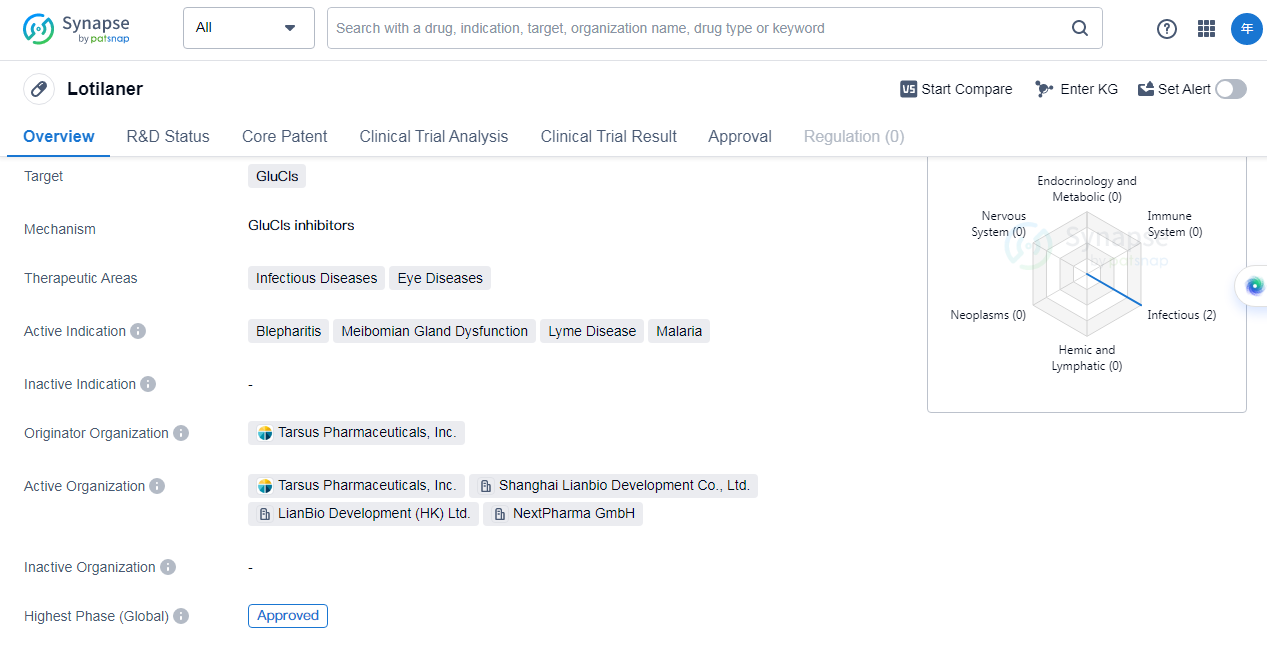
Lotilaner is a small molecule drug that targets glutamate-gated chloride channels(GluCls) and is primarily used in the treatment of infectious diseases and eye diseases. The drug has been approved for use in the United States and is expected to receive approval in China in the near future.
Lotilaner is indicated for the treatment of several conditions, including blepharitis, meibomian gland dysfunction, Lyme disease, and malaria. These conditions are all characterized by infectious or inflammatory processes that affect the eyes or other parts of the body. By targeting GluCls, Lotilaneris able to effectively combat the underlying causes of these diseases and provide relief to patients.
The drug was developed by Tarsus Pharmaceuticals, Inc., an organization specializing in the research and development of pharmaceutical products. Tarsus Pharmaceuticals has successfully brought Lotilaner through the various stages of clinical trials, with the drug currently in the highest phase of development, which is approved for use globally. In China, Lotilaner is in the phase 3 stage of development, indicating that it is nearing completion and may soon receive approval for use.
Lotilaner is expected to have a significant impact on the treatment of infectious and ocular diseases. With its approval in the United States and potential approval in China, the drug will be available to a wide range of patients in need. The first approval date for Lotilaner in the United States is set for July 2023, marking an important milestone in the drug's journey to market.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lotilaner: GluCls Inhibitors
GluCls inhibitors are a type of drug that target and inhibit the activity of GluCls receptors. GluCls receptors, also known as glutamate-gated chloride channels, are a type of ion channel found in the nervous system. They play a crucial role in mediating inhibitory neurotransmission by allowing the influx of chloride ions into neurons upon activation by the neurotransmitter glutamate.
By inhibiting GluCls receptors, GluCls inhibitors can modulate the excitability of neurons and affect various physiological processes. These inhibitors can have potential applications in the treatment of conditions such as epilepsy, anxiety disorders, and sleep disorders, as they can help regulate neuronal activity and restore the balance of neurotransmitters.
From a biomedical perspective, GluCls inhibitors are being studied and developed as potential therapeutic agents for neurological disorders. They are designed to specifically target GluCls receptors and modulate their function, offering a targeted approach to regulating neuronal activity. Further research is needed to fully understand the effectiveness and safety of GluCls inhibitors in different clinical settings.
Drug Target R&D Trends for Lotilaner
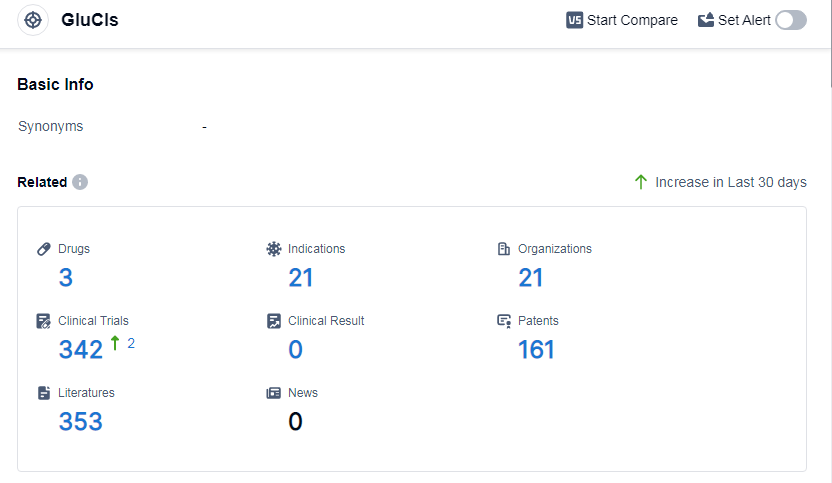
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 3 GluCls drugs worldwide, from 21 organizations, covering 21 indications, and conducting 342 clinical trials.
Based on the analysis of the provided data, the current competitive landscape of target GluCls shows several companies growing rapidly, with Tarsus Pharmaceuticals, Inc. and Medicines Development for Global Health Ltd being at the forefront. The highest stage of development is seen in Tarsus Pharmaceuticals, Inc. and Medicines Development for Global Health Ltd, with drugs in the approved stage. Indications such as onchocerciasis, rosacea, and elephantiasis, filarial have approved drugs under the target GluCls.
Small molecule drugs are progressing rapidly, with several drugs in the approved stage. The countries or locations with the highest development under the target GluCls are the United States, China, and Japan. The United States has the highest number of drugs in the approved stage.
Overall, the target GluCls presents a competitive landscape with companies at different stages of development and a focus on small molecule drugs. Further research is needed to understand the specific progress in China and the future development of target GluCls.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Lotilaner is a small molecule drug developed by Tarsus Pharmaceuticals, Inc. It targets GluCls and is indicated for the treatment of infectious and coular diseases such as blepharitis, meibomian gland dysfunction, Lyme disease, and malaria. The drug has received approval in the United States and is in the Phase 3 stage of development in China. Lotilaner is expected to have a significant impact on patient care and represents an important advancement in the field of biomedicine.