Kymera Therapeutics starts Phase 2 trial of KT-474 (SAR444656), a new IRAK4 degrader, on its first Hidradenitis Suppurativa patient
The initiation of patient dosing within the randomized Phase 2 clinical study in hidradenitis suppurativa evaluating KT-474 (SAR444656) was announced by Kymera Therapeutics, Inc. This company, which is currently at a clinical-stage, is working on developing a new category of drugs based on small molecule medicines through targeted protein degradation. In this study, the IRAK4 degrader, a first-of-its-kind investigational medication, is being tested.
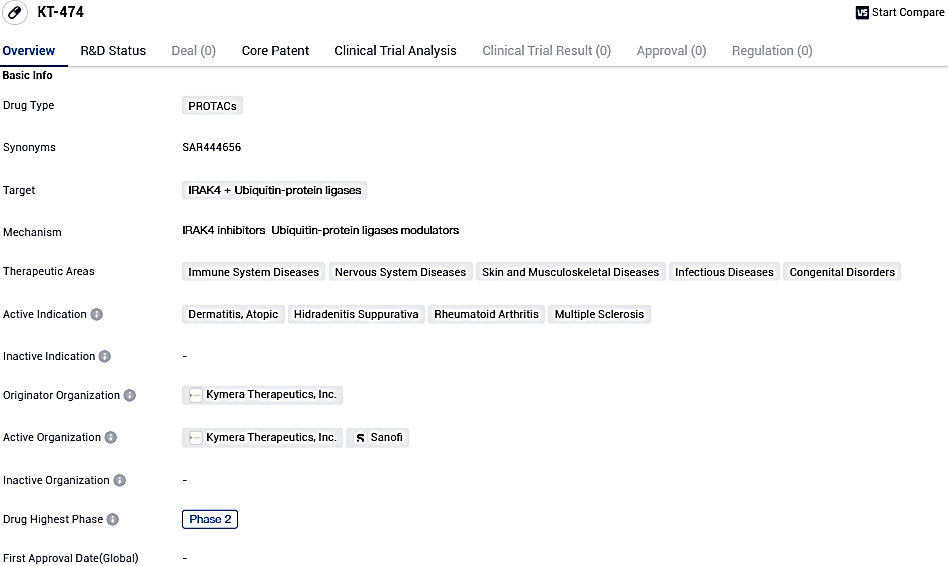
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The second phase of the study is set to examine the effectiveness, safety, pharmacokinetics, and biological impacts of KT-474 against a placebo in adult patients suffering from moderate or severe HS. Sanofi, Kymera's collaborator, is leading the second phase of the HS study and has instigated another randomized second phase trial for AD. As per the partnership agreement, the commencement of the first patient dosage in the HS trial generated a $40 million milestone payout. Initiating the dosage for the first AD trial patient will also lead to a milestone payment made to Kymera.
"The start of KT-474's first phase 2 trial dosage in HS is a key stride for the development of this molecule and a major accomplishment for Kymera, signaling the potential of protein degradation to revolutionize the treatment of intricate inflammatory diseases through small molecules," stated Nello Mainolfi, PhD, Founder, President, and CEO of Kymera Therapeutics.
Mainolfi further added, "Judging from the promising phase 1 outcomes of KT-474, we're optimistic that this molecule can provide HS patients with a potent and well-received oral medication. We anticipate releasing more updates as our partner Sanofi advances the program, including first patient dosage in the AD's second phase 2 trial within this quarter."
"Naturally, at Sanofi, we're thrilled about the promise that protein degraders bring in providing fresh treatments for immune and inflammatory diseases by selectively focusing on specific proteins and crucial pathways, like IRAK4," expressed Naimish Patel, MD, Head of Global Development, Immunology, and Inflammation, Sanofi. "HS remains a persistent and incapacitating skin ailment with a significant demand for novel treatment alternatives. We eagerly await the advancement of the phase 2 program in HS and AD."
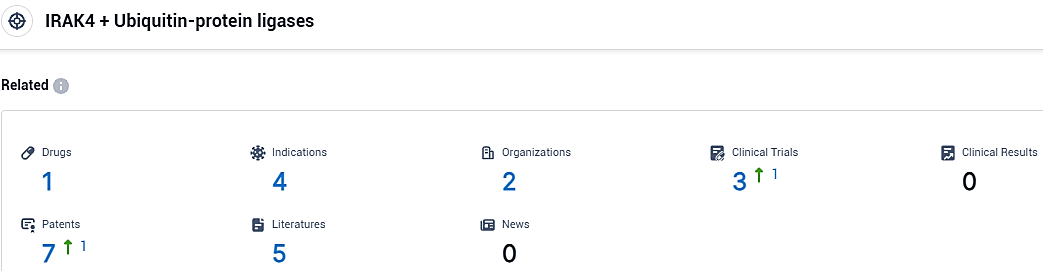
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 1, 2023, there are 1 investigational drugs for the IRAK4 and Ubiquitin-protein ligases target, including 4 indications, 2 R&D institutions involved, with related clinical trials reaching 3, and as many as 7 patents.
KT-474, an orally administered IRAK4 degrader, is being developed for addressing complex IL-1R/TLR-driven inflammatory diseases where there's potential to notably improve the existing treatment standards.Presently, the safety and effectiveness of KT-474 are undergoing assessment in blinded, placebo-controlled, random Phase 2 clinical trials involving adults suffering from moderate to severe HS and AD. Collaborations have been established between Sanofi and Kymera for the development of KT-474 excluding the areas of oncology and immune-oncology. The Phase 2 examinations are being directed by Sanofi.