FDA Approves Vertex's Suzetrigine for Moderate-to-Severe Acute Pain
Vertex Pharmaceuticals Incorporated stated that the U.S. Food and Drug Administration has approved its New Drug Application for suzetrigine, an investigational, oral, selective NaV1.8 pain signal inhibitor indicated for treating moderate-to-severe acute pain. Suzetrigine could become the initial new medication class for managing acute pain in more than two decades.
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
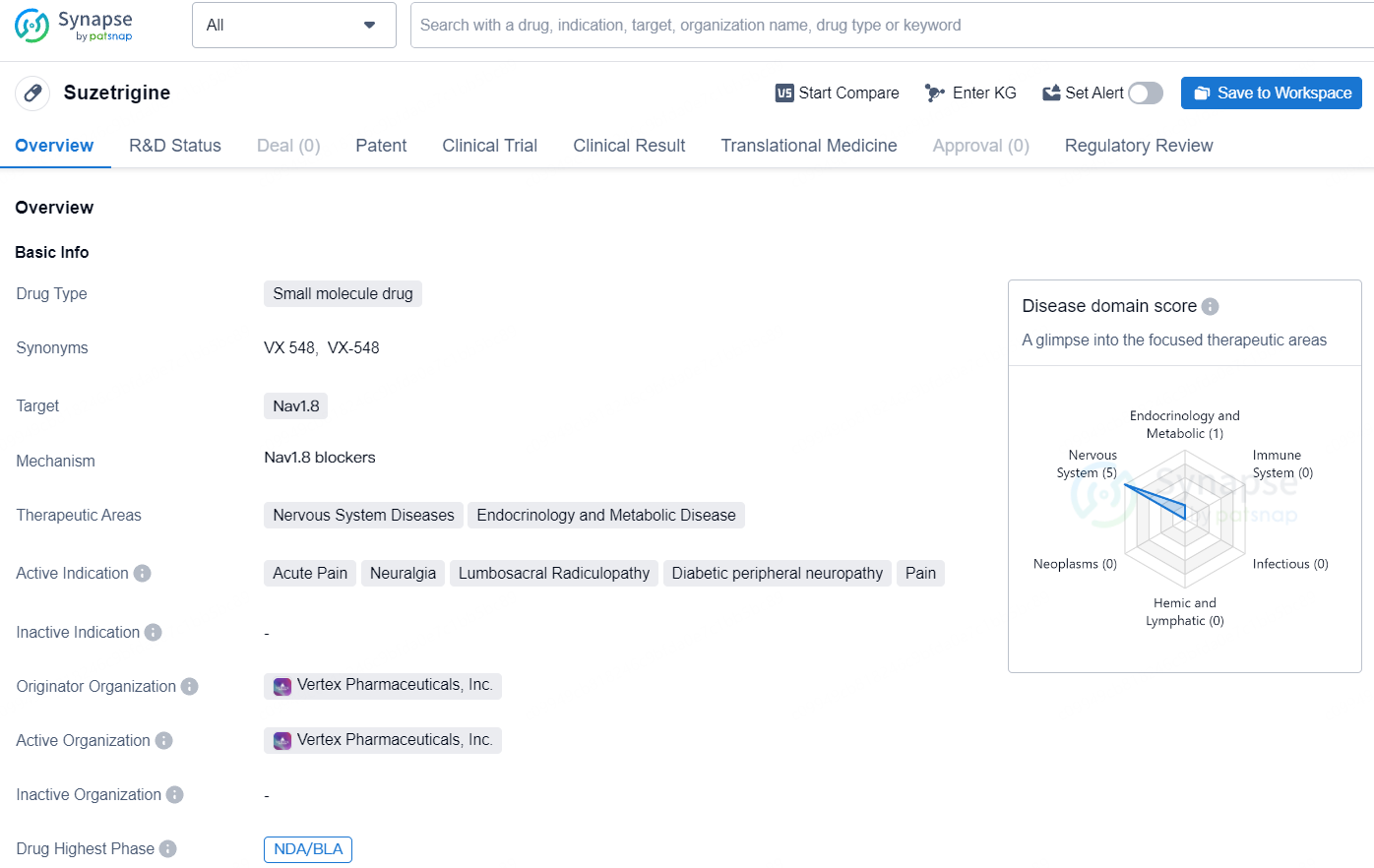
The FDA has given suzetrigine priority review and set a Prescription Drug User Fee Act target action date of January 30, 2025. Suzetrigine has received FDA Fast Track and Breakthrough Therapy designations for treating moderate-to-severe acute pain.
"The FDA's acceptance of suzetrigine's filing today is a significant step forward in bringing this innovative non-opioid analgesic to the millions of patients facing moderate-to-severe acute pain annually in the U.S.," stated Nia Tatsis, Ph.D., Executive Vice President, Chief Regulatory and Quality Officer at Vertex.
"The priority review granted by the FDA highlights the substantial unmet need in acute pain management, and the filing brings us closer to addressing the gap between medications with decent tolerability but limited effectiveness and opioid medications with recognized risks, including the potential for addiction," Nia Tatsis continued.
"During my 24-year medical career, I have witnessed the urgent requirement for new non-opioid therapies to treat pain. Many individuals today are either not receiving adequate treatment, experiencing adverse effects from existing therapies, or avoiding pain medications entirely out of concerns about opioid dependency," stated Scott Weiner, M.D., M.P.H., Vertex Acute Pain Steering Committee Chair, Associate Professor of Emergency Medicine at Harvard Medical School, and Attending Emergency Physician at Brigham and Women's Hospital.
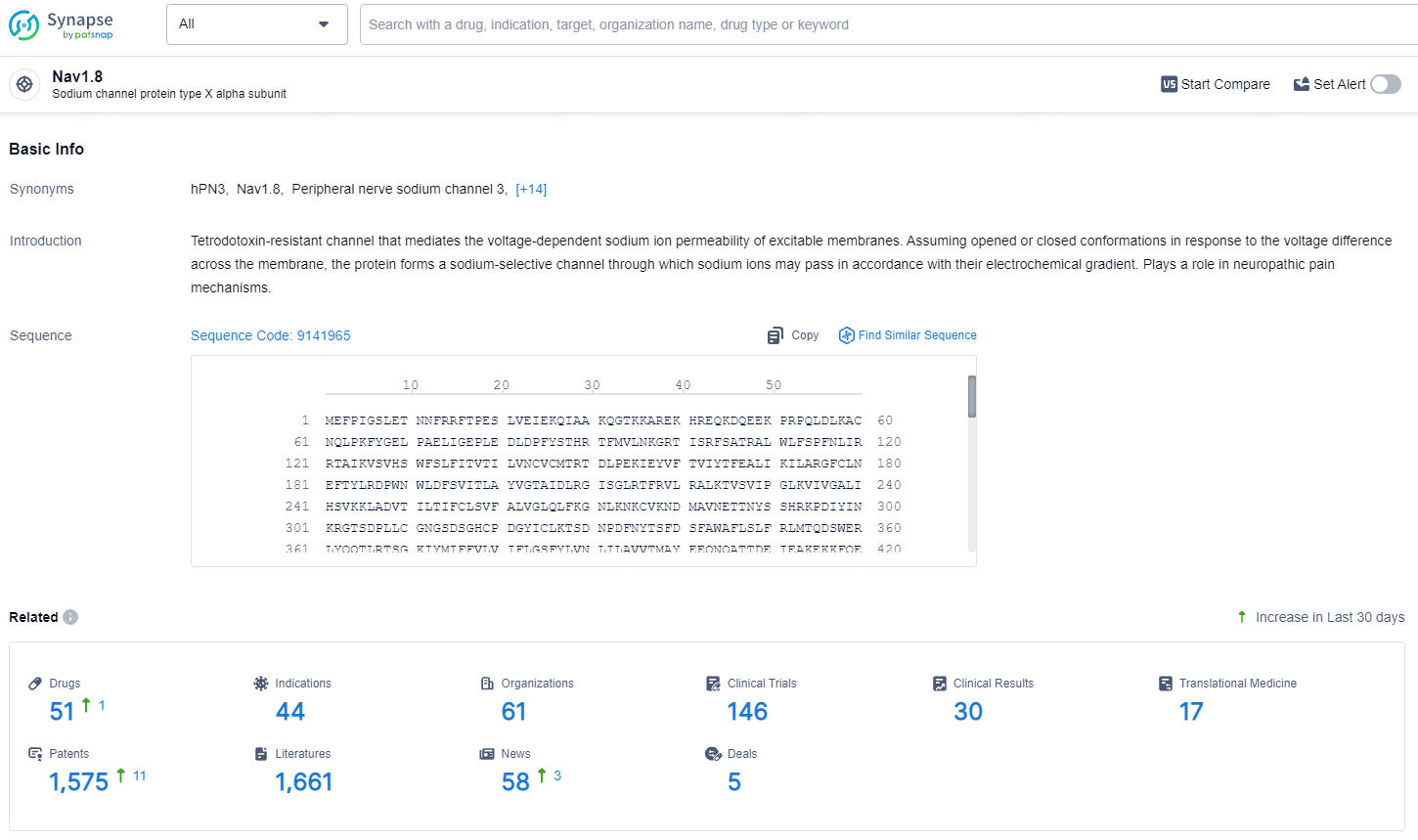
Suzetrigine (previously known as VX-548) is an investigational oral, selective NaV1.8 pain signal inhibitor with a high level of specificity for NaV1.8 in comparison to other NaV channels. NaV1.8 is a voltage-gated sodium channel found exclusively in peripheral pain-sensing neurons (nociceptors) responsible for transmitting pain signals.
In a Phase 2 study involving patients with pain linked to diabetic peripheral neuropathy, a form of chronic peripheral neuropathic pain, suzetrigine showed promising outcomes and a well-tolerated profile. Vertex's strategy involves the targeted inhibition of NaV1.8 using small molecules to develop a novel class of pain signal inhibitors aiming to offer effective pain relief without the shortcomings of current therapies, such as the addictive nature of opioids.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of August 2, 2024, there are 51 investigational drugs for the NaV1.8 targets, including 44 indications, 61 R&D institutions involved, with related clinical trials reaching 146, and as many as 1575 patents.
Suzetrigine is a novel small molecule drug with the aim of addressing various nervous system and metabolic diseases, specifically targeting Nav1.8. With its designation as a Fast Track and Breakthrough Therapy, the drug shows promise in potentially offering new treatment options for patients suffering from conditions such as acute pain, neuralgia, lumbosacral radiculopathy, diabetic peripheral neuropathy, and pain. Its current highest phase of development, NDA/BLA, indicates that it is progressing through the regulatory pathway for potential approval and commercialization.