Context Therapeutics has revealed preclinical studies, illustrating the distinct and potent effect of its Claudin 6-focused bispecific antibody CTIM-76
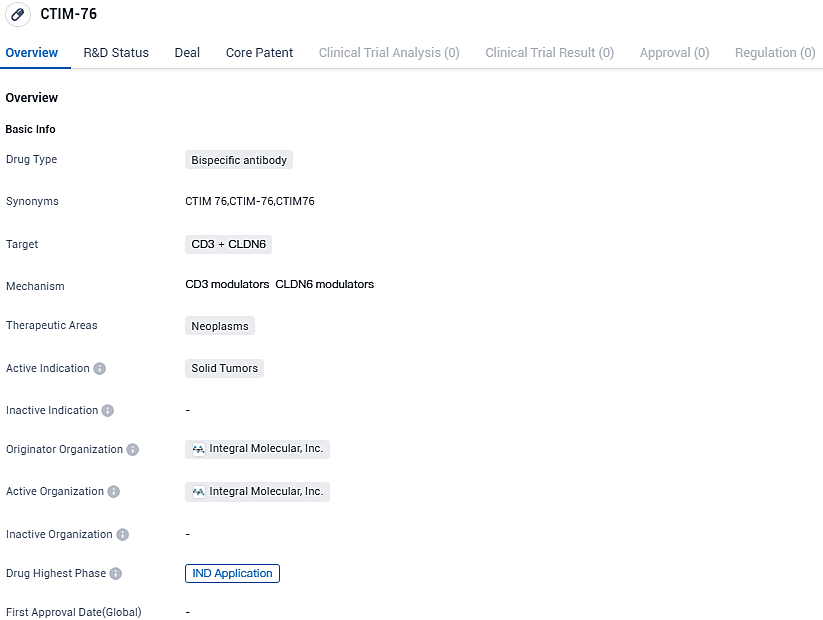
Context Therapeutics Inc., a company focusing on biopharmaceuticals and developing drugs for solid tumors, has revealed promising preclinical results for its research asset, CTIM-76. This asset is a Claudin 6 (CLDN6) x CD3 T-cell engaging bispecific antibody in the preclinical stage.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Martin Lehr, Context's CEO, stated, "In the U.S., about 70,000 individuals are estimated to have CLDN6-positive metastatic solid tumors pathologies, and currently, there are no sanctioned therapies that target them accurately." He followed this by expressing optimism about the potential CTIM-76 has exhibited in initial in vivo experiments.
This contributes to our understanding of its selectivity and power seen in former in vitro studies and highlights CTIM-76's capacity to induce complete tumor remission at varying dosage levels. The efficacy of clinical-stage compounds underscores CTIM-76's capability to manage potential issues tied to target density and toxicity that are encountered with initial-generation strategies.
The set of information will be showcased during the poster segment of the 38th Annual Meeting of the Society for Immunotherapy of Cancer held on November 3, 2023, in San Diego. To see the abstract, details can be found on the SITC meeting's official website.
"In this year's SITC meeting, the preclinical data will shed light on CTIM-76's potential to focus on CLDN6," Lehr disclosed. "Given all these findings, we consider CTIM-76 a hopeful candidate for targeting CLDN6, and we anticipate to file an Investigational New Drug Application towards the end of the first quarter in 2024."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
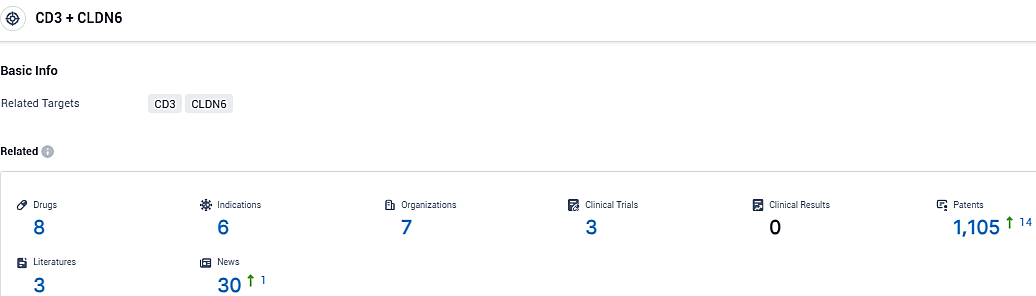
According to the data provided by the Synapse Database, As of November 6, 2023, there are 8 investigational drugs for the CD3 and CLDN6 target, including 6 indications, 7 R&D institutions involved, with related clinical trials reaching 3, and as many as 1105 patents.
CTIM-76 is a bispecific antibody drug and is intended for the treatment of solid tumors. The drug is currently in the IND application phase, signifying its progress towards clinical trials.