Corbus Starts First Human Trial of CRB-601 for Solid Tumors
Corbus Pharmaceuticals Holdings, Inc. (NASDAQ: CRBP), a company with a diversified portfolio in oncology and obesity, has announced the initiation of dosing in the Phase 1 segment of the clinical trial for CRB-601, aimed at treating patients with advanced solid tumors (NCT06603844). CRB-601 is a monoclonal antibody designed to target latent TGFβ activation by inhibiting the integrin αVβ8. Preclinical studies have shown that CRB-601 effectively counteracts tumor immune exclusion and boosts the efficacy of immune checkpoint inhibitors in vivo.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
"CRB-601 presents an innovative upstream method for regulating TGFβ signaling, and this dose escalation study may provide significant insights, including the potential to observe the immune system's activation and the possibility of tumor response," stated Dr. Dominic Smethurst, MA, MRCP, Chief Medical Officer of Corbus.
"Initiating dosing of CRB-601 in a clinical trial is a crucial milestone, moving us closer to grasping its clinical potential and setting the stage for progress in a promising area of immunotherapy," remarked Dr. Jeffrey M. Clarke, MD, Associate Professor of Medicine, Associate Director of the Thoracic Oncology Clinical Research Program at Duke Cancer Institute, Raleigh, NC, and a lead investigator in the CRB-601 study.
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
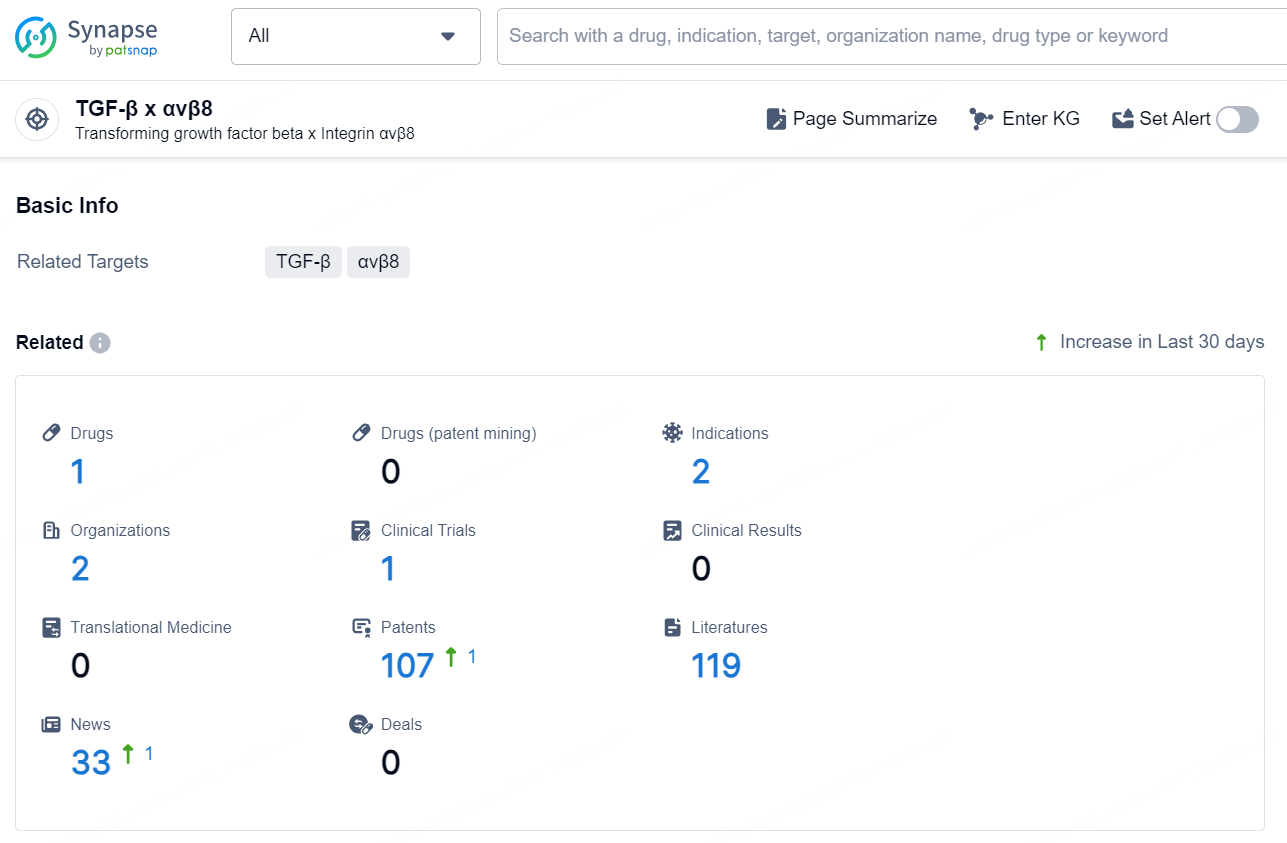
According to the data provided by the Synapse Chemical, As of December 12, 2024, there are 1 investigational drug for the TGF-β x αvβ8 target, including 2 indications, 2 R&D institutions involved, with related clinical trial reaching 1, and as many as 107 patents.
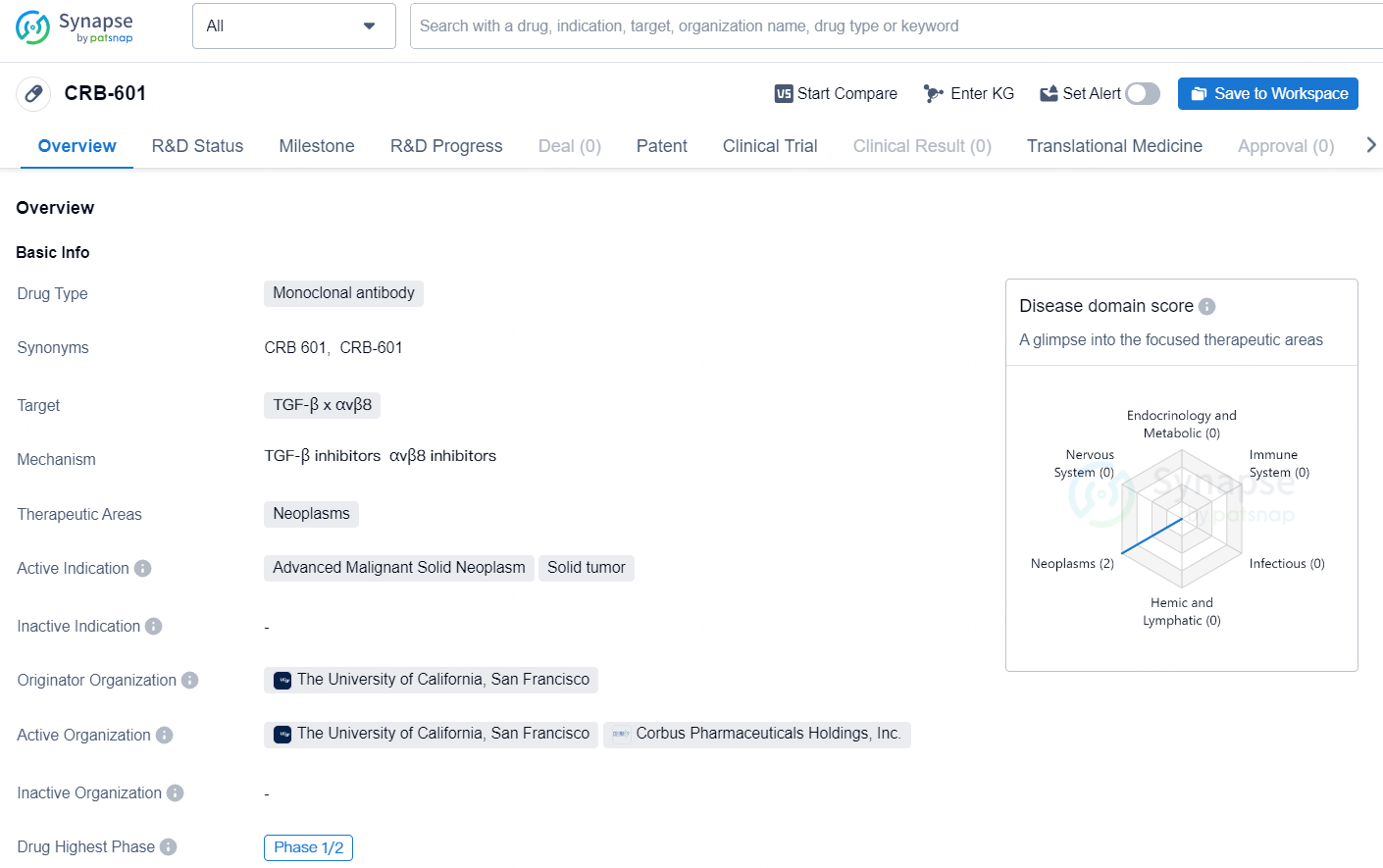
CRB-601 is a monoclonal antibody drug that targets TGF-β and αvβ8 and is primarily focused on the therapeutic areas of neoplasms. The active indications for this drug are advanced malignant solid neoplasm and solid tumor. The originator organization of CRB-601 is The University of California, San Francisco. The drug is currently in Phase 1/2, which represents the stage of development in the global pharmaceutical market.