AbbVie's IL-23 antibody Risankizumab completes regulatory filing for new indications in Europe and the United States
On August 29, 2023, AbbVie submitted a new indication application to the US FDA and European EMA for Skyrizi (Risankizumab) for the treatment of moderate to severe ulcerative colitis (UC) in adults. This application is based on the data from the phase 3 clinical trials INSPIRE and COMMAND, which support Risankizumab as an induction and maintenance therapy. Analyses showed that the trial achieved the primary clinical remission endpoint (according to the adaptive Mayo score) and the key secondary endpoint.
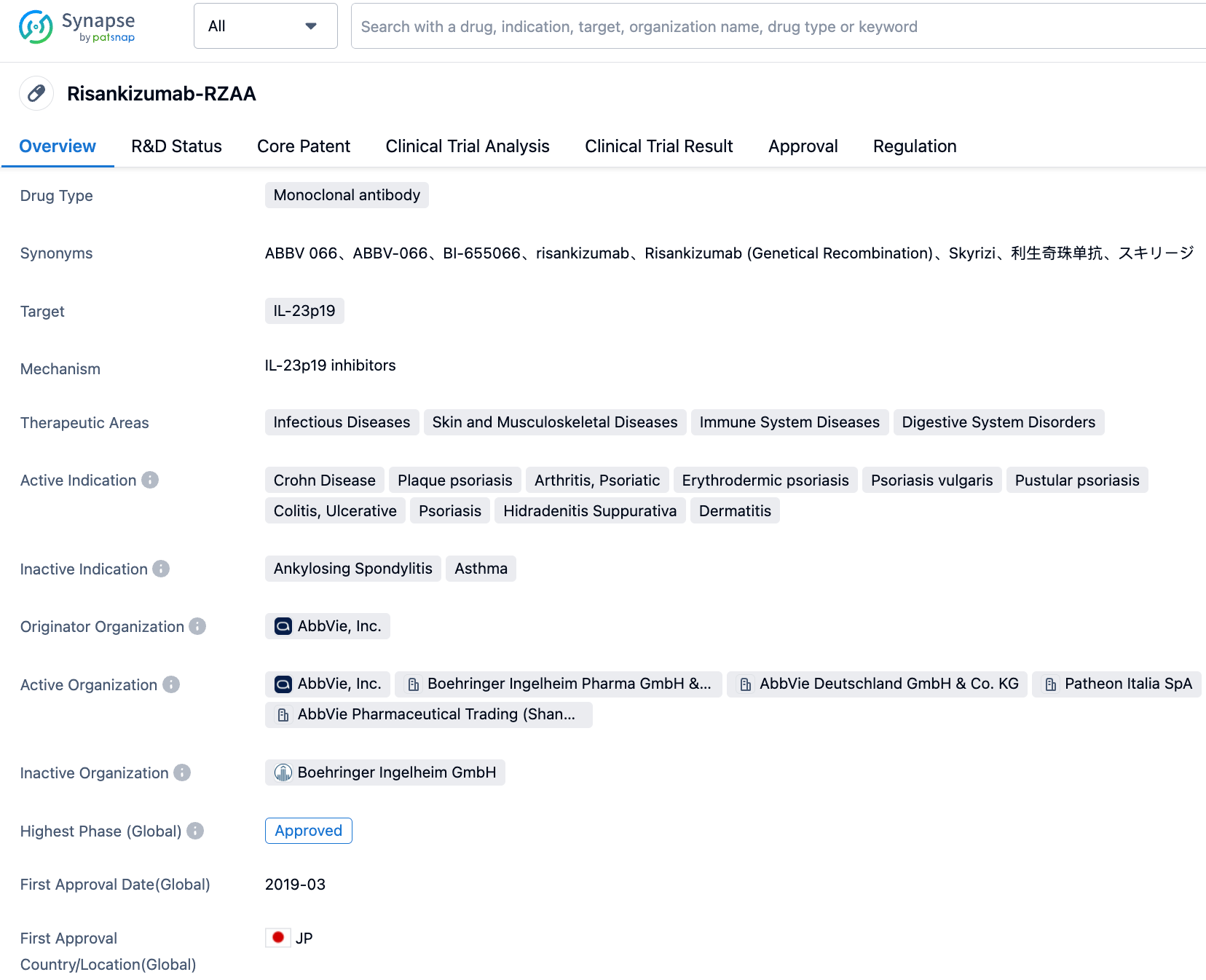
Risankizumab is an interleukin-23 (IL-23) inhibitor that selectively blocks IL-23 by binding to its p19 subunit. IL-23 is a cytokine involved in the inflammation process and is believed to be associated with many chronic immune-mediated diseases. Risankizumab was first approved in April 2019, with two approved indications by the FDA and EMA being psoriatic arthritis and plaque psoriasis. In its first year of marketing, Skyrizi brought in $355 million in sales, which increased to $1.59 billion in the second year. By 2022, the global sales had reached $5.165 billion.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The analysis showed that a significantly higher proportion of patients treated with 180 mg and 360 mg risankizumab achieved clinical remission at week 52: 40% and 38% respectively, compared to 25% in the control group (p<0.01). Among patients treated with 180 mg and 360 mg risankizumab, 51% and 48% respectively demonstrated an improvement upon endoscopy, while only 32% of patients in the control group showed improvement (p<0.001). Furthermore, 43% and 42% of patients treated with 180 mg and 360 mg risankizumab, respectively, showed histological endoscopic mucosal improvement, significantly higher than the control group patients (23%, p<0.001). In addition, a significantly higher proportion of patients treated with 180 mg and 360 mg risankizumab also achieved clinical remission without corticosteroids at week 52 (p<0.01).
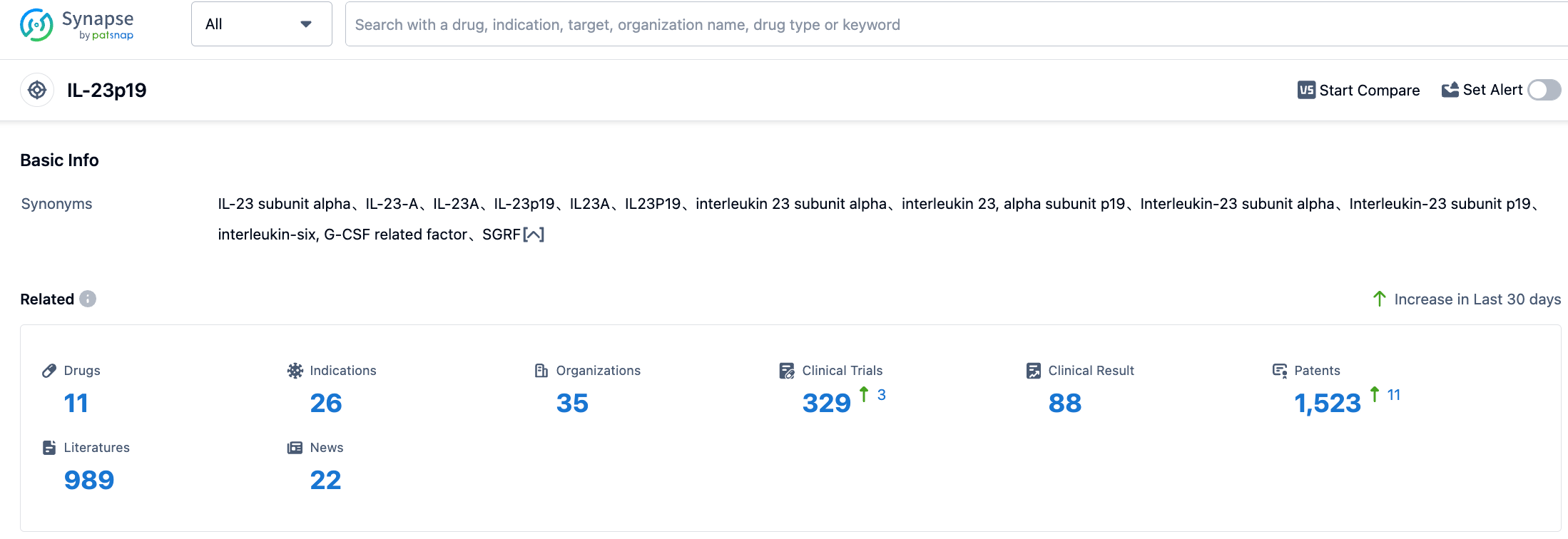
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the information disclosed by the SYNAPSE database, as of August 30, 2023, there are a total of 38 drugs under development targeting IL-23, covering 28 indications, with 66 research institutions involved, encompassing 270 related clinical trials, and as many as 10,848 patents... Since its launch in 2019, Risankizumab has continued to maintain a high-speed growth trend in sales. It has yet to be approved for the market in China among the top three global pharmaceutical markets. It is anticipated that its future entry into the Chinese market will create a new sales miracle.