BridgeBio's TTR stabilizer acoramidis achieves primary endpoint in Phase 3 clinical trial
Recently, BridgeBio Pharma announced that its investigational treatment, acaracides, achieved its primary endpoint in the phase 3 clinical trial, ATTRibute-CM, for patients with Transthyretin-Mediated Amyloidosis with Cardiomyopathy (ATTR-CM). The therapy significantly reduced the risk of patient mortality and hospitalization due to cardiovascular disease. The detailed data was presented at the 2023 European Society of Cardiology (ESC) conference.
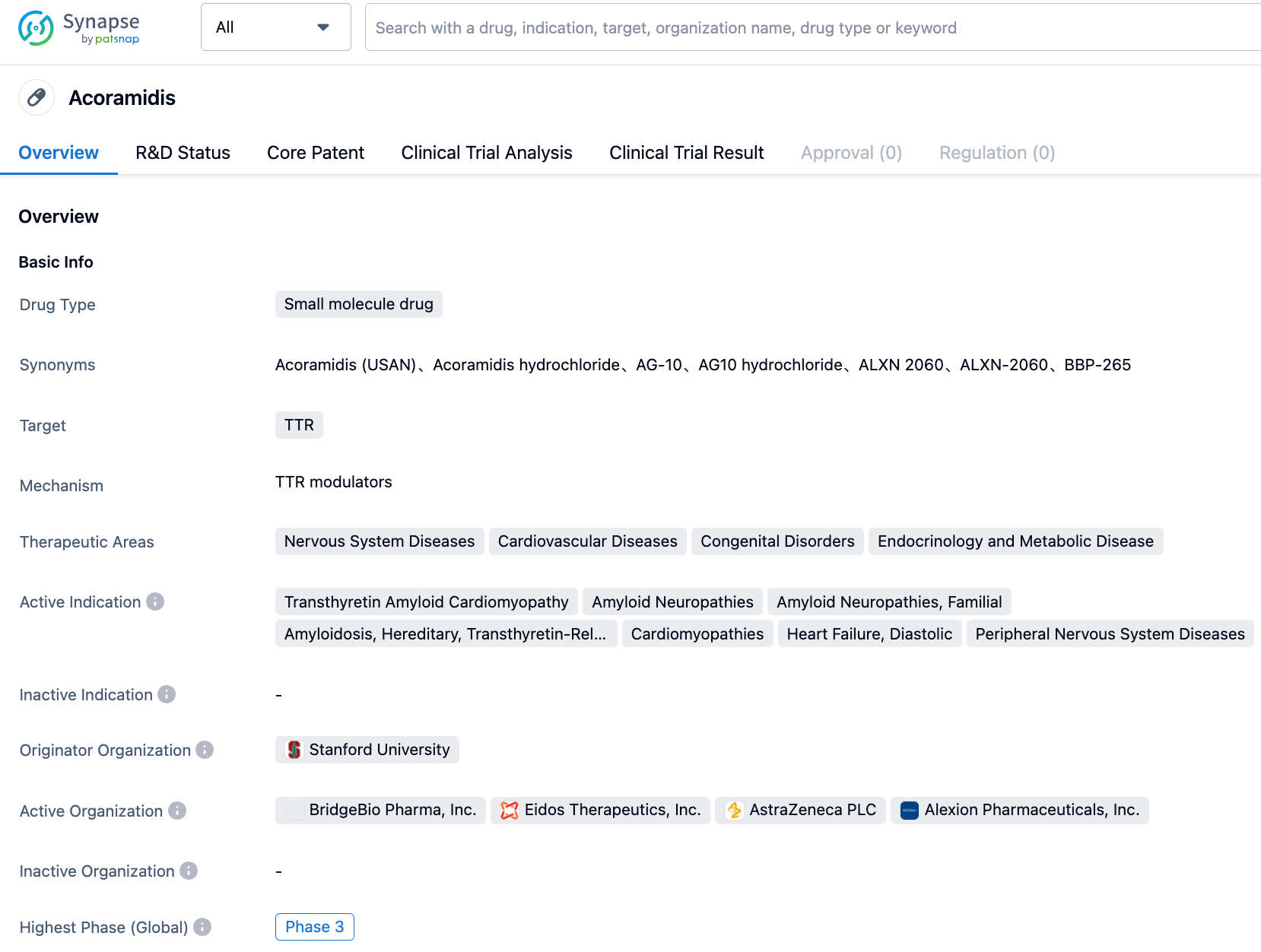
Acoramidis is a novel, orally administered, potent Transthyretin (TTR) stabilizer developed by BridgeBio Pharma. It is designed to effectively stabilize tetrameric TTR, preventing the cascade of molecular events that lead to TTR Amyloidosis (ATTR) from the outset. Acoramidis is able to mimic a natural variation of the TTR gene (T119M) considered a "rescue mutation" as it has been shown to prevent or reduce ATTR in individuals with TTR gene mutations.
ATTR Amyloidosis is a rapidly progressing, debilitating rare disease caused by misfolded TTR leading to the accumulation of abnormal amyloid proteins in various tissues, including nerves, heart, and the gastrointestinal (GI) tract. Until now, therapeutic options were limited for patients with ATTR Amyloidosis accompanied by cardiomyopathy, presenting a significant unmet medical need.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The results of this Phase 3 clinical trial showed a significant improvement in the primary endpoint with high statistical significance (from the stratified analysis in order of precedence: all-cause mortality, then hospitalization frequency associated with cardiovascular disease, then changes from baseline in N-terminal pro b-type natriuretic peptide, then changes from baseline in 6-minute walk distance), with a Win ratio of 1.8 (p<0.0001). In contrast to results observed in previous studies with TTR stabilizers, consistency was observed across all pre-specified disease subgroups in terms of resistance to cardiovascular-related hospitalization (CVH) at 30 months. The absolute values observed in all-cause mortality (ACM), cardiovascular mortality (CVM) and CVH indicated that over a 30-month period, there was a higher survival rate and lower hospitalization rate compared to results observed in the company's previous ATTR-CM control study. The survival rate of 81% in the Acoramidis group approached the survival rate in an age-matched U.S. database (about 85%).
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
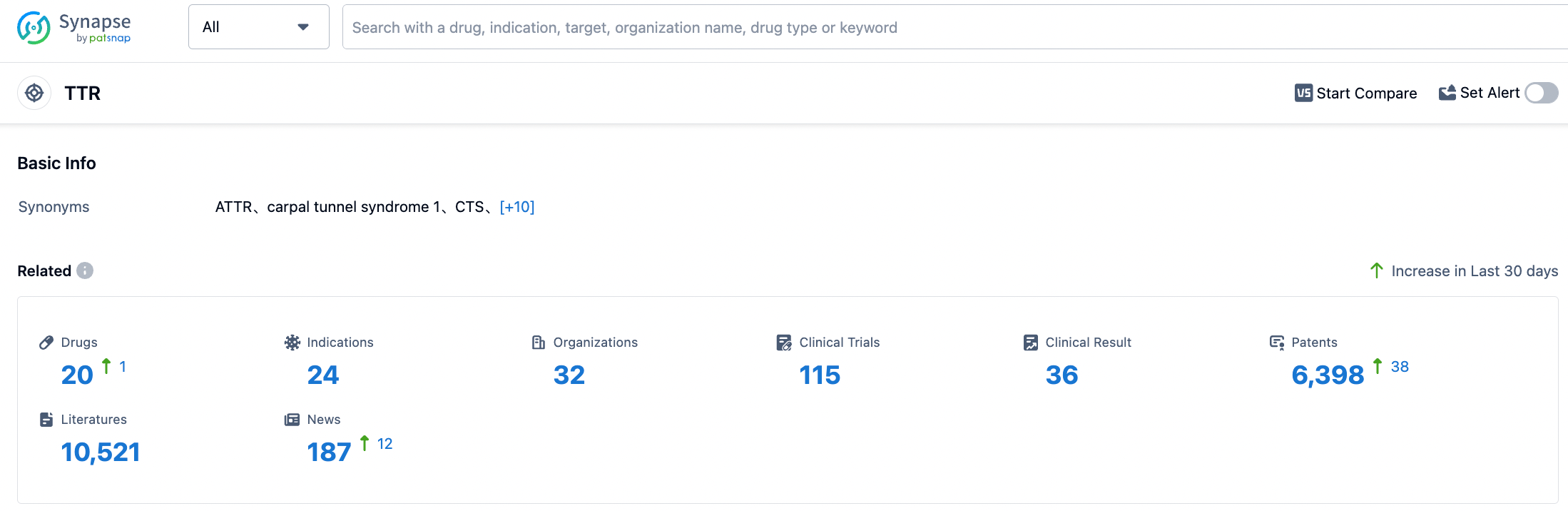
According to the information disclosed by the Synapse database, as of August 30, 2023, there are a total of 20 investigational drugs targeting TTR. These drugs are applicable to 24 different indications, are studied by 32 institutions, and are involved in 115 clinical trials, with as many as 6416 related patents. BridgeBio Pharma is expected to submit a New Drug Application (NDA) for acoramidis to the US FDA by the end of this year, potentially bringing new therapeutic options to patients.