Cue Biopharma presents positive Phase 1 results for CUE-101 (Head and Neck Cancer) and CUE-102 (Wilms' Tumor 1) at SITC 2023
Cue Biopharma, Inc., a clinical-phase bio-pharma firm producing an innovative range of T cell engagers to selectively control cancer-specific T cells, has revealed new promising findings from its continued, completely enrolled Phase 1 trials. This examination reviews the application of their lead (IL-2) based T cell engager, CUE-101, alone and mixed with KEYTRUDA in treating patients with relapsed/metastatic HPV+ head and neck squamous cell cancer.
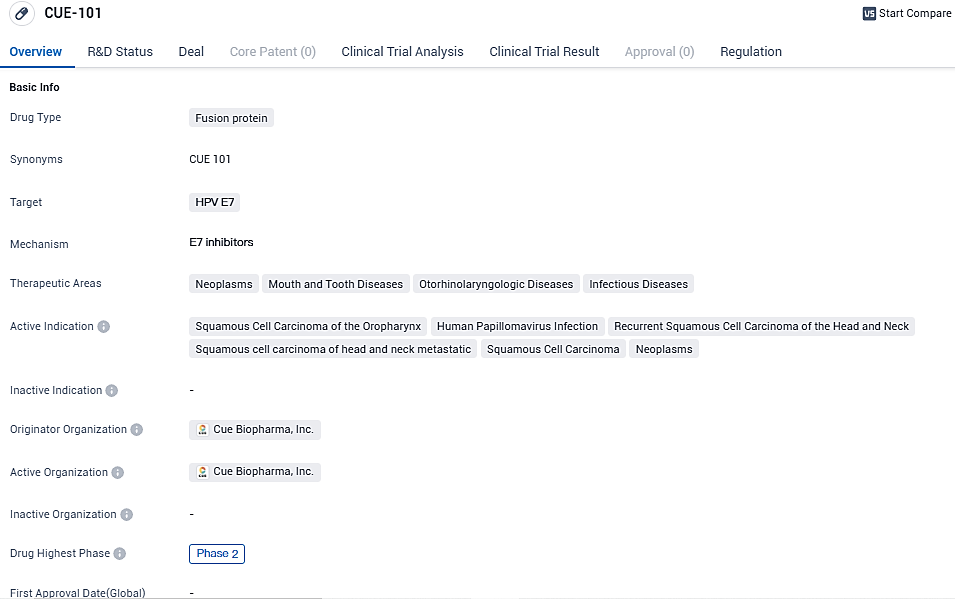
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The company will also share fresh clinical evidence from the currently conducting Phase 1 investigation of its secondary candidate, CUE-102, intended for Wilms’ Tumor 1 positive recurrent/metastatic cancer treatment. “I find the clinical improvement in patients observed so far promising, and also the progressive enhancement of information from the CUE-101 clinical trials,” said Christine Chung, M.D., Department Head, Head and Neck-Endocrine Oncology, Moffitt Cancer Center, and a main investigator involved in the clinical trial.
“Significant extension in overall survival beyond 20 months in monotherapy denotes a prominent progress of clinical benefit unlike the present care standard for this group with advanced and unresponsive disease.” The increased overall response rate reported so far along with pembrolizumab, relative to the historic response rate for pembrolizumab only, offers great hope for first line patients.
Chronic and unmet medicinal needs persist for more potent and less harmful treatments for people with recurrent/metastatic head and neck cancer, and the results from the CUE-101 trial illustrate the potential to meet this requirement.
The CEO of Cue Biopharma, Dan Passeri, commented, “The clinical evidence produced so far, from both our CUE-101 monotherapy and combination experiments in HPV+ R/M HNSCC, showcase what we think is a top-tier method to immune regulation, and a robust route forward to achieve the complete potential of activating the patient's own immune response against cancer.
We are satisfied with our achievements so far and eagerly anticipate continued progress across our platform as we keep working on promising cancer therapies.” CUE-101 is engineered to stimulate and enlarge HPV16 tumor-specific T cells through presenting a pair of signals or “cues” to T cells. Cue #1 integrates the HPV E7 protein, carried by HPV-induced cancer cells, offering selectivity via interaction with the HPV-specific T cell receptor. Cue #2 comprises an engineered IL-2 variant to encourage T cell activity.
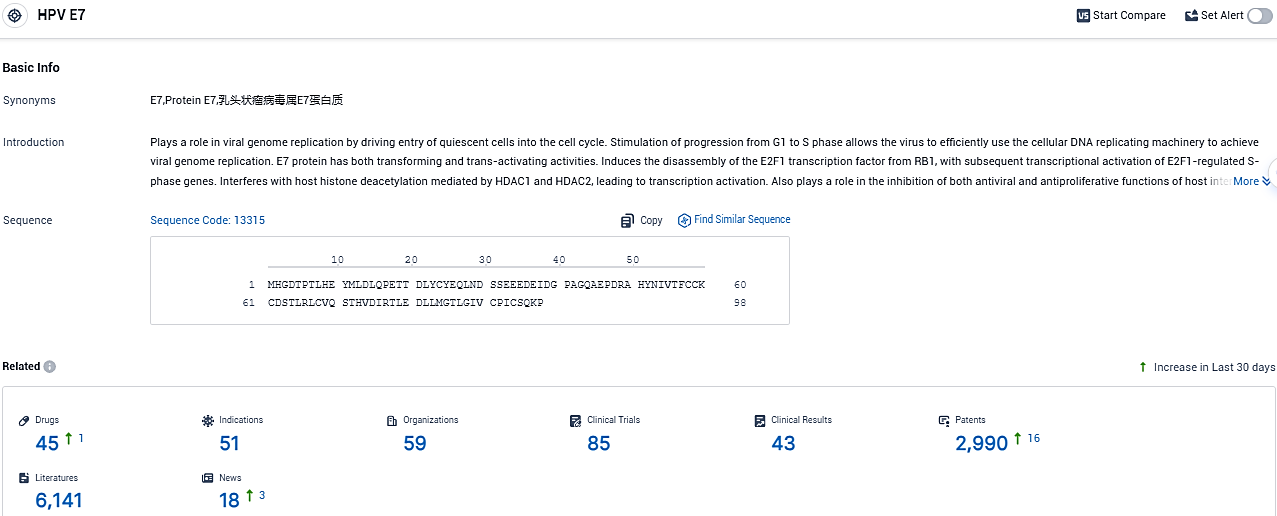
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 11, 2023, there are 45 investigational drugs for the HPV E7 target, including 51 indications, 59 R&D institutions involved, with related clinical trials reaching 85, and as many as 2990 patents.
CUE-101 is currently being evaluated in a fully enrolled Phase 1 open-label, dose escalation and expansion study, for the treatment of HPV16+ driven recurrent/metastatic head and neck squamous cell carcinoma in second line and beyond patients as a monotherapy, and as a first line therapy in combination with pembrolizumab (KEYTRUDA).