Cullinan Oncology begins Phase 1 trial of CLN-617, a novel biologic fusing IL-2 and IL-12 cytokines
Cullinan Oncology, Inc., a company specializing in the development of precise cancer treatments without preference for treatment type, has disclosed the initiation of patient treatment with its investigational drug CLN-617 during an early-stage clinical study.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
We are conducting an inaugural trial for humans, spanning multiple centers with a non-blinded approach, aiming to assess the safety profile, pharmacokinetic behavior, pharmacodynamic effects, and initial effectiveness of CLN-617 both as a monotherapy and when used in tandem with pembrolizumab. Pembrolizumab serves as an inhibitor targeting the programmed death receptor-1, and the study involves participants who are dealing with advanced forms of solid malignancies.
CLN-617 represents a pioneering approach to cytokine treatment, ingeniously fusing human IL-2, IL-12, a domain that binds to collagen, and an element that augments molecular size into a unified therapeutic agent devised for direct injection into tumor sites. The purpose of these distinctive features is to ensure the cytokines remain at the tumor site post-injection, aiming to enhance both the safety and effectiveness of the treatment.
In the exploration using preclinical models, the direct tumor administration of CLN-617 initiated a widespread systemic immune reaction, which led to the shrinkage of remote tumors not subjected to the injection and created a T cell memory response that contributed to augmented long-term survival rates.
"Checkpoint inhibitors have significantly transformed the field of immuno-oncology. However, their efficacy can be limited in instances where tumors possess a deficient quantity of immune-specific T cells, commonly characterized as 'cold' tumors. IL-2 alongside IL-12 are cytokines that, in synergy, activate an immune assault on cancer cells. This could potentially lead to an influx of T cells penetrating the tumor, their subsequent activation, and proliferation, effectively rendering the tumor 'hot'," expressed Jeffrey Jones, MD, MPH, MBA, the Chief Medical Officer at Cullinan Oncology. "We are eager to collaborate with clinical researchers to delve into the capabilities of CLN-617."
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
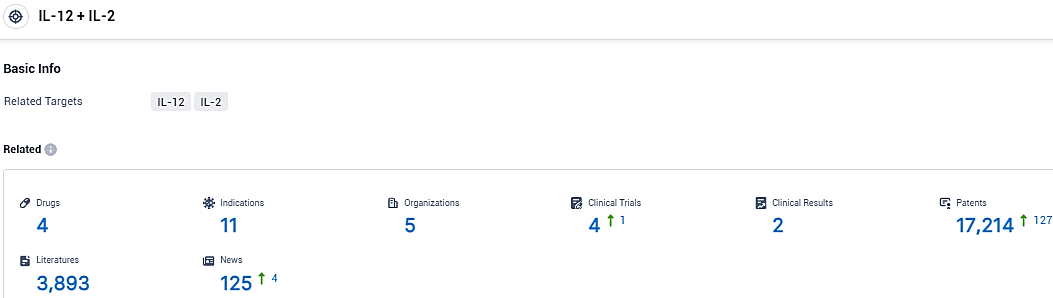
According to the data provided by the Synapse Database, As of December 27, 2023, there are 4 investigational drugs for the IL-12 and IL-2 target, including 11 indications, 5 R&D institutions involved, with related clinical trials reaching 4, and as many as 17214 patents.
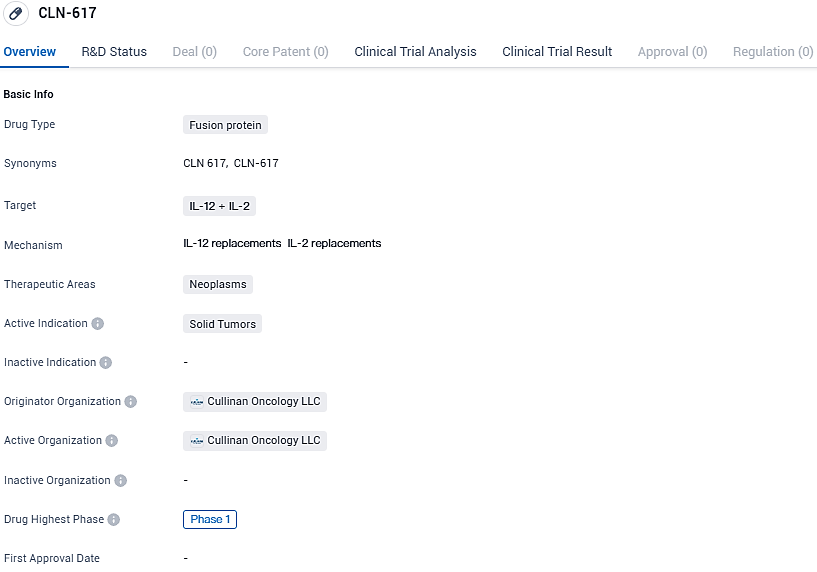
CLN-617 is a fusion protein drug that targets IL-12 and IL-2 and is being developed for the treatment of solid tumors. The development of this drug holds promise for improving the treatment options available for patients with solid tumors.