Curadev Unveils CRD3874: A Novel STING-Activating Allosteric Compound
Curadev is thrilled to announce the launch of their innovative compound, CRD3874—an exceptional allosteric small molecule that uniquely stimulates the STING pathway, different from others that target the cGAMP site within STING. The US FDA has granted authorization for an intravenous version of this pioneering molecule to be employed in initial human trials, specifically targeting individuals with advanced-stage solid tumors.
👇Unlock in-depth information about this drug - its R&D Status, Core Patent, Clinical Trials, and Global Approval Status. Click on the image below and explore the latest data immediately.
At the Society for Immunotherapy of Cancer's annual event held in San Diego, United States, in November 2023, Curadev disclosed outcomes from preclinical research which demonstrated that systematic administrations of a drug via the bloodstream induced strong immune responses leading to the eradication of tumors in various cancer models within rodents. Additionally, the data indicated that a biweekly intravenous administration of CRD3874 on its own was received without significant adverse effects in primate subjects.
The data indicate that CRD3874 may have substantial potential as a therapeutic for human use, providing a rationale for further clinical trials involving cancer sufferers. Differing from other STING agonists competing over the CDN-binding site, CRD3874 distinguishes itself due to a unique pharmacological profile and a favorable safety profile likely owing to its non-competitive, allosteric interaction with the target.
The data were presented in a poster which included Dr. Ciara Kelly, a specialist from the Memorial Sloan Kettering Cancer Center, as a co-author. As the primary investigator of the First-in-Human (FIH) trial, Dr. Kelly's expertise contributed to the research findings. The research and development efforts under the leadership of Monali Banerjee, the VP of R&D at Curadev who also spearheads the STING-related projects and serves as the primary poster author, further underscore the potential for CRD3874. Banerjee expressed optimism about the initial experimental outcomes hinting at CRD3874's capabilities in activating the immune system against advanced cancers.
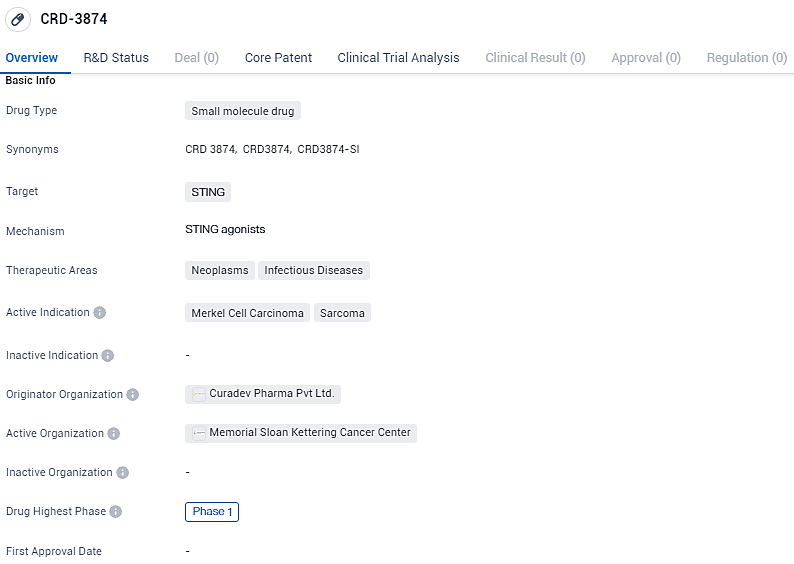
CRD3874 is currently under scrutiny for its efficacy against specific cancerous conditions, notably Merkel Cell Carcinoma and various forms of Sarcoma. Merkel Cell Carcinoma is an uncommon yet rapid skin cancer variant, whereas Sarcoma encompasses a broad category of cancers arising from structural tissues such as bone, muscle, or connective tissue.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
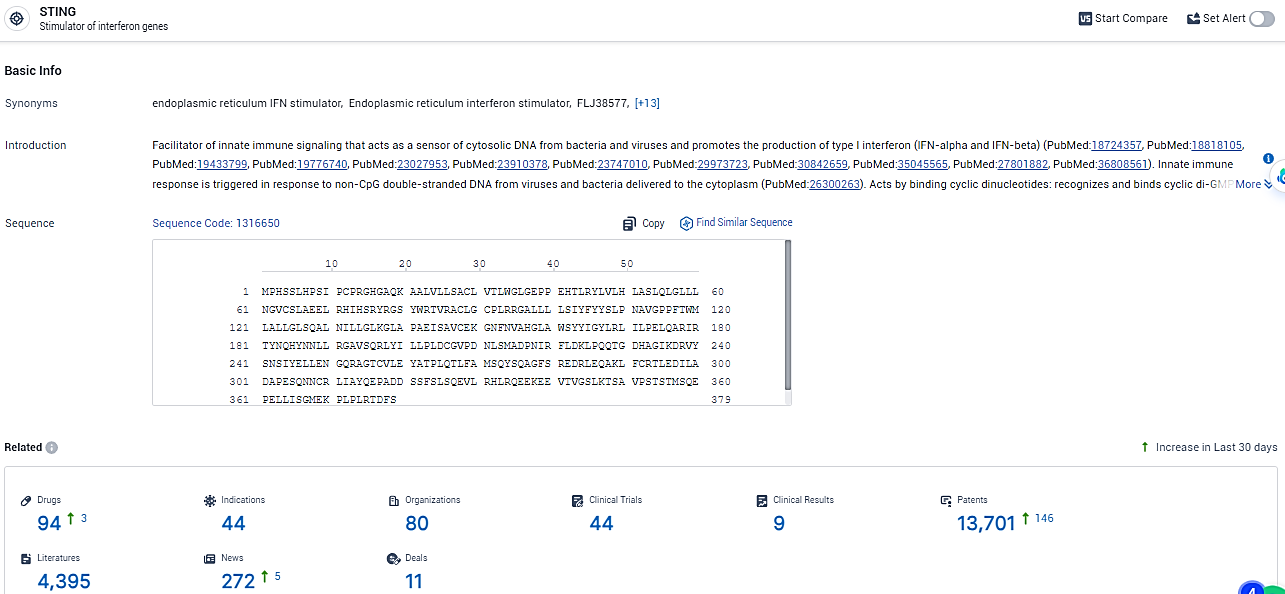
According to the data provided by the Synapse Database, As of February 22, 2024, there are 94 investigational drugs for the STING target, including 44 indications, 80 R&D institutions involved, with related clinical trials reaching 44, and as many as 13701 patents.
CRD-3874 is a small molecule drug that targets STING, a protein involved in immune response regulation. The therapeutic areas for CRD-3874 include neoplasms and infectious diseases. As of the latest available information, CRD-3874 has reached Phase 1 of clinical trials, which is the initial stage of testing in humans. Phase 1 trials primarily focus on evaluating the safety, dosage, and potential side effects of a drug. This suggests that CRD-3874 is still in the early stages of development and further research is required to determine its efficacy and potential benefits for patients.