Nuvig Launches Early Stage Trials for Advanced Immunomodulator NVG-2089
Nuvig Therapeutics, Inc., has revealed the commencement of the initial human administration of its unique compound, NVG-2089, which is in development for the management of individuals suffering from inflammatory myopathies and critical autoimmune skin conditions. Additionally, Nuvig disclosed that NVG-2089 has received expedited review status from the FDA for its advancement in the treatment of bullous pemphigoid.
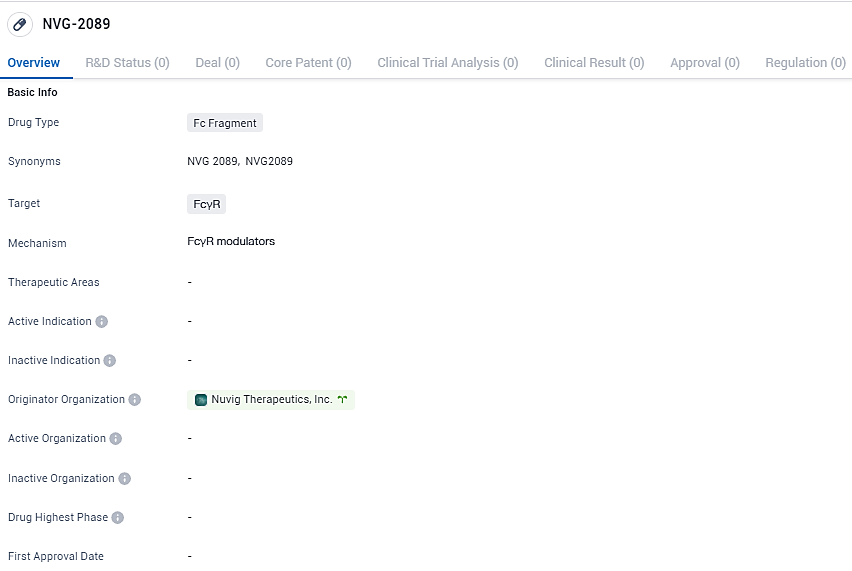
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
NVG-2089 represents an innovative, engineered, non-immunosuppressive agent that specifically interacts with type II Fc receptors to initiate a natural regulatory response, aiming to alleviate autoimmunity imbalances. This compound has shown potent anti-inflammatory responses in preclinical trials involving animal models with hyperactive B cells, T cells, and certain myeloid cells within the immune system. In preclinical toxicology testing, NVG-2089 has been well received with no significant adverse effects observed.
Julie Anne Smith, the CEO of Nuvig Therapeutics, expressed enthusiasm about moving NVG-2089 into further clinical testing. She emphasized the company's eagerness to test the compound in various autoimmune conditions where it may offer substantial advantages in terms of safety and effectiveness when compared to current treatment options.
The initial focus of the clinical study is to investigate the safety profile and the tolerability of NVG-2089 in human subjects. The study will also look into the drug's pharmacokinetic and pharmacodynamic attributes. Once the Phase 1 trial is concluded, Nuvig Therapeutics intends to launch studies to establish the clinical benefits in patients affected by inflammatory muscle diseases and severe autoimmune skin disorders.
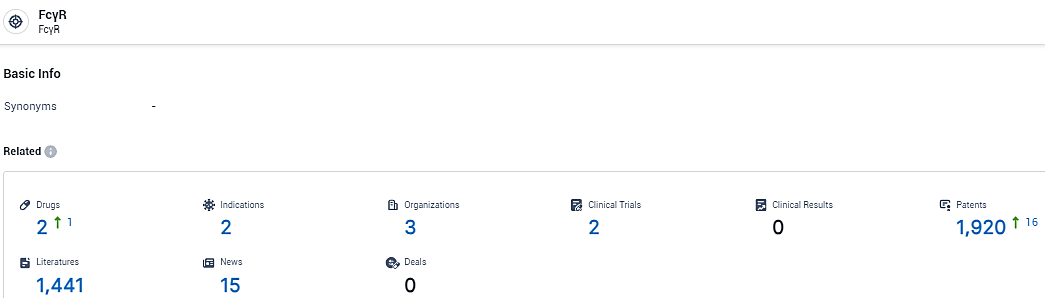
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
According to the data provided by the Synapse Database, As of February 20, 2024, there are 16 investigational drugs for the FcγR target, including 34 indications, 13 R&D institutions involved, with related clinical trials reaching 24, and as many as 339 patents.
NVG-2089 is an Fc Fragment drug that targets FcγR. As an expert in the pharmaceutical industry, further research and analysis are required to fully understand the drug's potential applications and impact in the field of biomedicine.