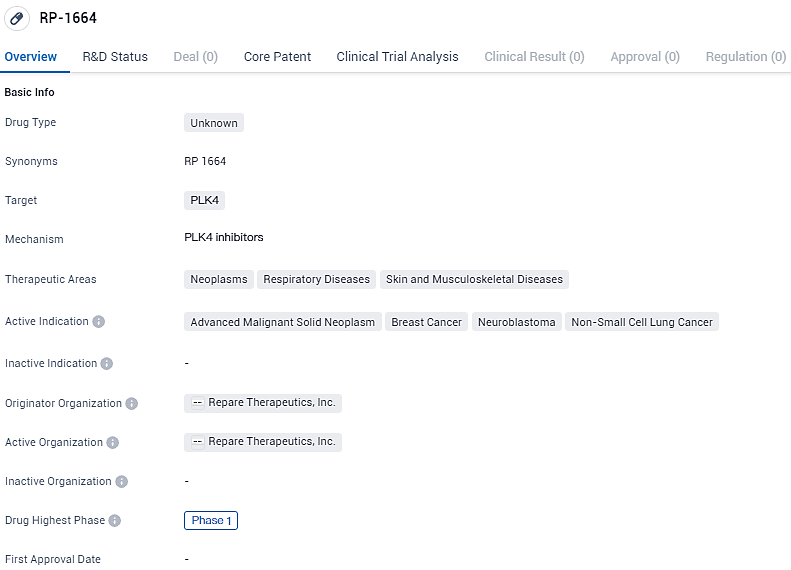
First Participant Receives Initial Dose of RP-1664 in Repare Therapeutics' Early-Stage Study
Repare Therapeutics Inc., a foremost company specializing in precision oncology in the clinical trial stage, has reported that the initial participant has received treatment within their LIONS Phase 1 study. This investigation is centered around RP-1664, which is considered to be at the forefront of its class as an orally administered, highly specific targeting polo-like kinase 4 inhibitor, designed for use as a standalone therapy. It is intended for the treatment of solid tumors with a high presence of TRIM37 in patients who are adults and adolescents.
👇Explore more about this drug by clicking the image below. Gain detailed insights into its R&D Status, Core Patent, Clinical Trials and Global Approval Status. Stay informed and updated.
"RP-1664 has shown significant suppression of tumor growth as well as tumor reduction across a variety of solid tumors with high TRIM37 expression and neuroblastoma xenograft models. This success was seen both in-house and through a partnership with the Children's Hospital of Philadelphia," remarked Maria Koehler, MD, PhD, who holds the position of Executive Vice President and Chief Medical Officer at Repare.
Maria Koehler elaborated, "Upon establishing a safety profile in the LIONS trial, our plan is to expedite the progression to a combined Phase 1/2 trial targeting children at high risk of recurrent neuroblastoma. This particular patient group generally suffers from neuroblastoma characterized by genetic TRIM37 alterations, for which there are few viable treatments. RP-1664 is the third drug candidate developed in-house by Repare, demonstrating the innovative nature of our research platform."
The LIONS trial signifies a pioneering, multi-site, open-label Phase 1 investigation designed to scrutinize RP-1664's safety, dynamics within the body, and initial therapeutic effects. The trial expects to recruit close to 80 subjects who suffer from advanced-stage solid tumors that have been carefully selected based on molecular characteristics, including those with TRIM37 amplifications or other genetic anomalies. The essential goals of the trial are to set the optimal doses and intervals for RP-1664 administration, establish tolerability and safety, and preliminarily gauge its ability to counteract tumors.
RP-1664 is regarded as a groundbreaking, orally administered PLK4 inhibitor selectivity targeting tumors. It leverages a synthetic lethal interaction caused by the overexpression or amplification of TRIM37 in such tumors. These tumors depend on PLK4 for generating centrioles during the S-phase of cellular division—a phase marked by heightened availability of TRIM37, an E3 ligase known to deplete pericentriolar material.
👇Explore the most recent advancements in drug research, indications, organizations, clinical trials, results, and patents related to this target by clicking the image link below. Dive in to gain deeper insights!
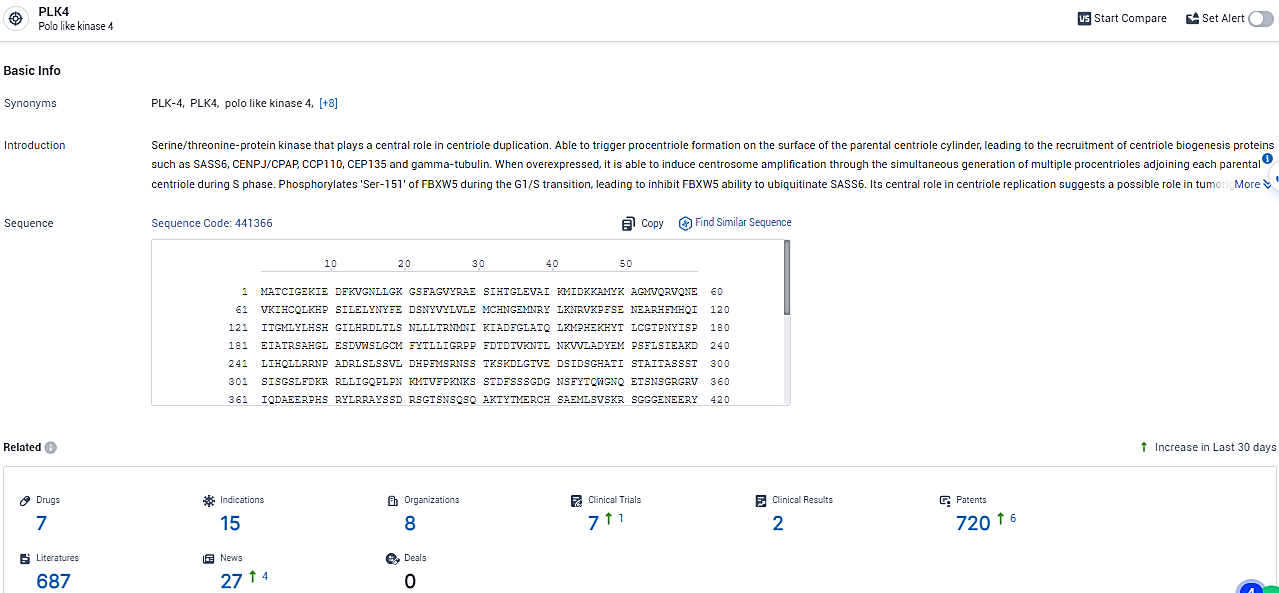
According to the data provided by the Synapse Database, As of February 22, 2024, there are 15 investigational drugs for the PLK4 target, including 8 indications, 7 R&D institutions involved, with related clinical trials reaching 2, and as many as 720 patents.
RP-1664 is a drug targeting PLK4 and has shown potential in treating neoplasms, respiratory diseases, and skin and musculoskeletal diseases. Its active indications include advanced malignant solid neoplasm, breast cancer, neuroblastoma, and non-small cell lung cancer. Developed by Repare Therapeutics, Inc., RP-1664 is currently in Phase 1 of clinical trials, indicating its early-stage development and potential for further evaluation and advancement in the pharmaceutical industry.