Deciphering the cGAS-STING Pathway: Unlocking the Secrets of Aging and Inflammation
Aging is a natural process, but it brings about a series of physical changes that can leave us vulnerable to illness. One such change is the increase of low-grade inflammation, a characteristic of aging that leads to deterioration and potential damage to our bodies. The exact mechanisms that drive this inflammation and its effects on natural aging remain a mystery to scientists.
cGAS–STING and ageing-related inflammation
On August 2, Professor Andrea Ablasser's team at the Swiss Federal Institute of Technology in Lausanne published an article titled "cGAS–STING drives ageing-related inflammation and neurodegeneration" in Nature.
The article identified the cGAS-STING pathway as a driver of inflammation in tissues and the brain associated with aging. The researchers suggested blocking cGAS-STING signaling as a potential strategy to slow aging-induced neurodegenerative processes.
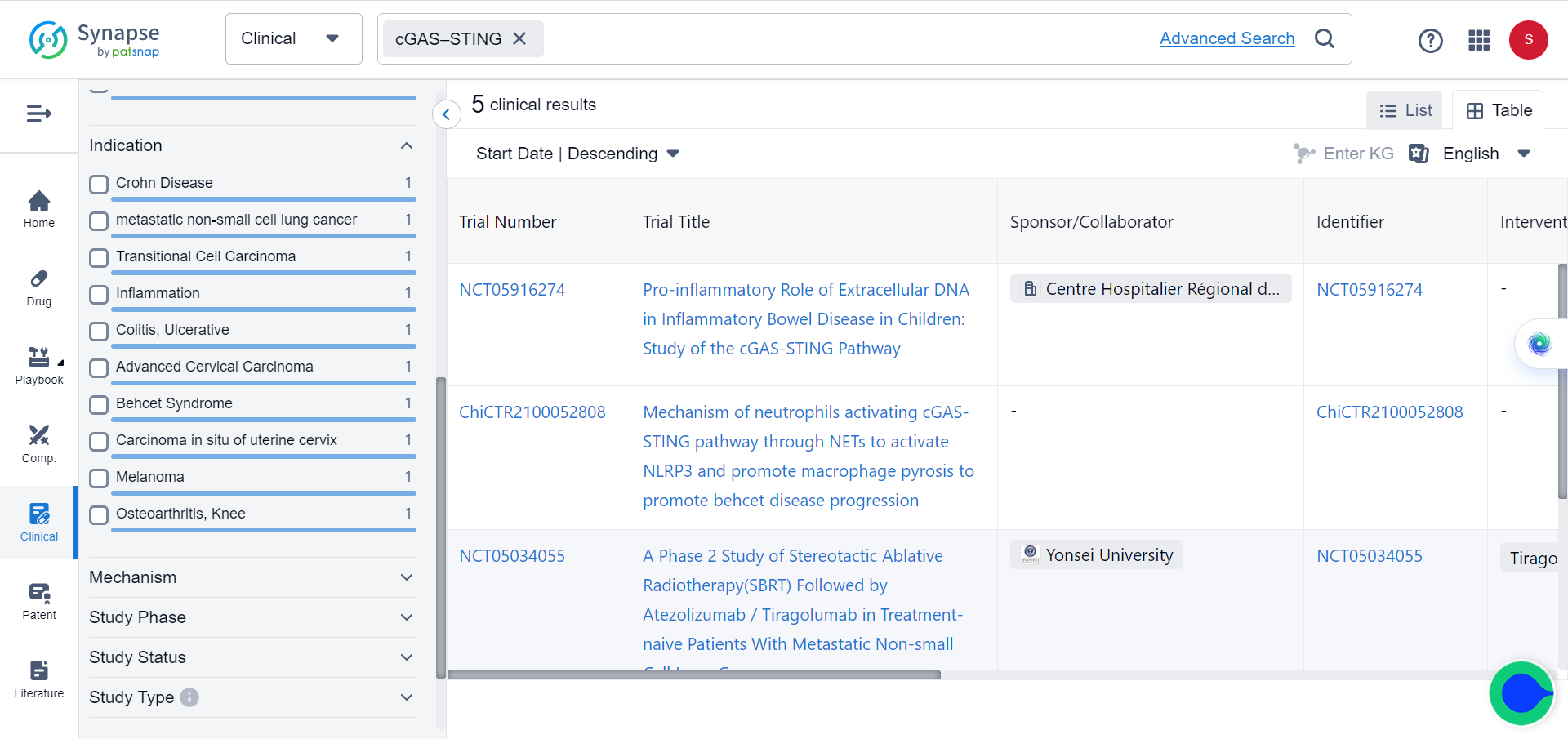
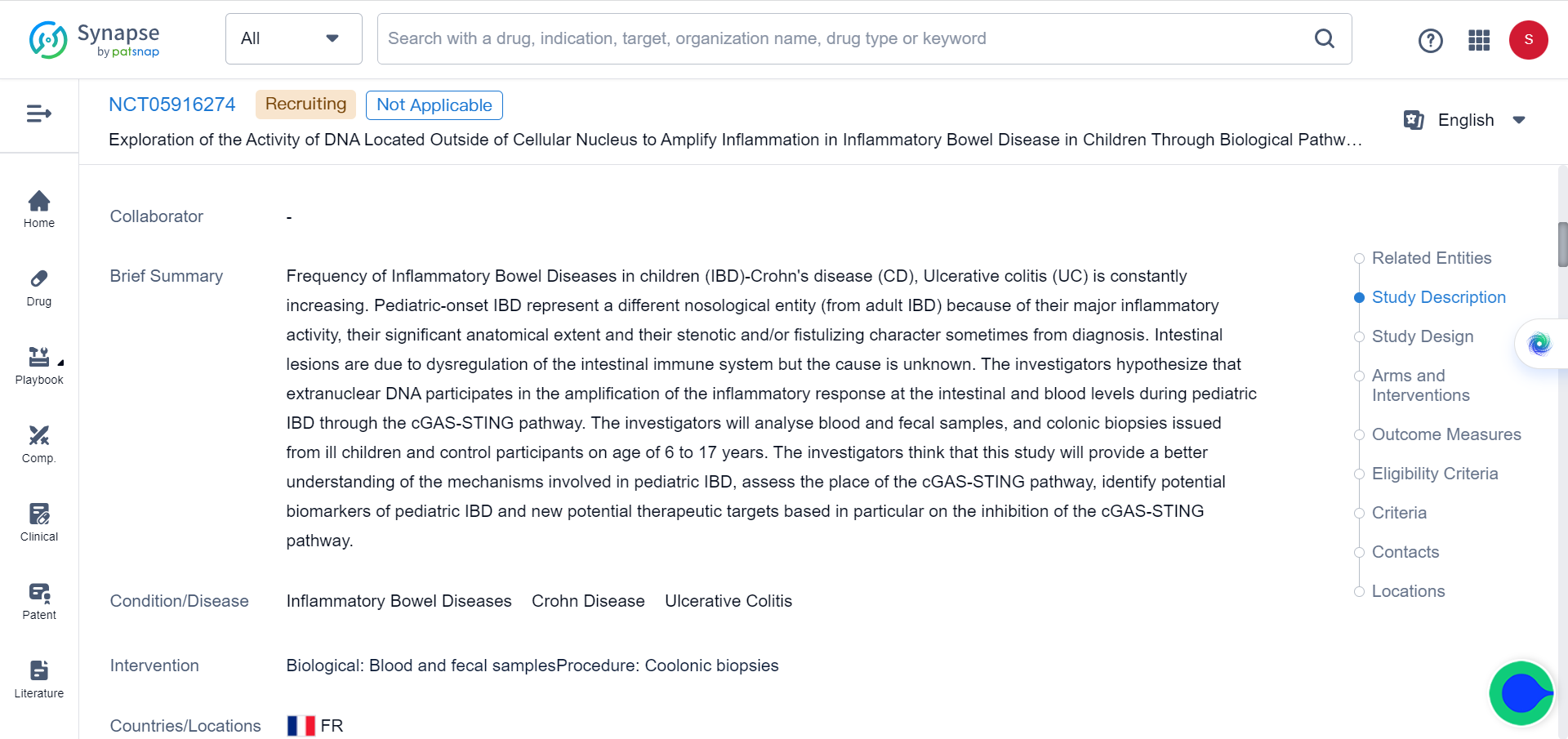
cGAS-STING is a molecular signaling pathway involving two proteins, cyclic GMP-AMP synthase (cGAS) and stimulator of interferon genes (STING). Once activated, cGAS/STING triggers an immune response to defend against viral and bacterial infections. Currently there are 5 clinical trials involving cGAS-STING pathway for a variety of conditions, including one investigating the pro-inflammatory role of extracellular DNA in inflammatory bowel disease in children, as indicated in Synapse database:
Previous studies had already suggested the cGAS-STING pathway's role in cellular aging. However, it remained unclear if the cGAS-STING pathway directly caused cellular aging in tissues or age-related inflammation and dysfunction.
To study the pathway's role in age-related phenotypes, the researchers first tested whether inhibiting STING with a selective small molecule inhibitor could suppress inflammatory responses in aging cells and human tissues. They found that inhibiting STING prevented inflammation in senescent cells.
Next, by blocking STING in old mice, they found various age-related immune characteristics diminished significantly. Cellular cytokines decreased along with reduced accumulation of inflammatory cells in the kidneys of STING-inhibited animals. Similarly, aged STING knockout mice showed lower levels of aging immune markers compared to aged wildtype mice.
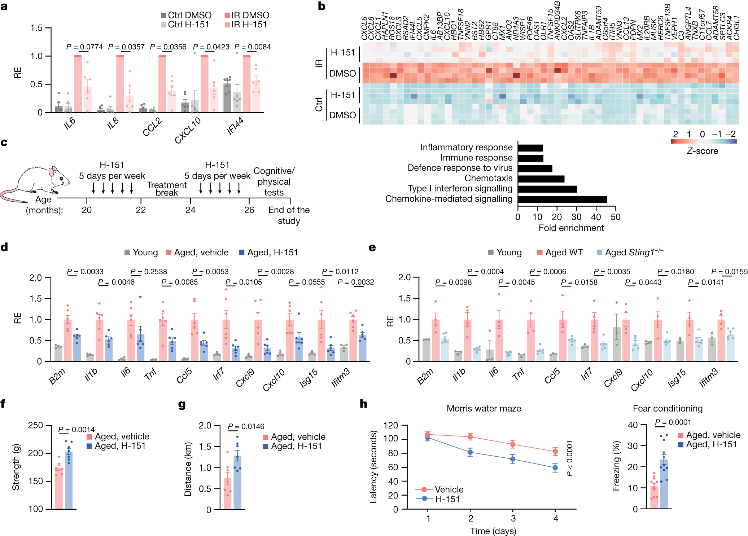
The researchers then explored whether reducing STING-dependent inflammation would affect physical and cognitive functions in old mice. They found that treated old mice had improved muscle strength and endurance. In hippocampus-dependent learning and memory tasks, spatial and associative memory also significantly improved with STING inhibition. The results indicated STING is an important driver of age-related inflammation, promoting cognitive decline in both peripheral and central nervous systems.
The researchers then focused on STING's effects on brain aging. STING inhibition reduced microglial proliferation in old mice. It also protected hippocampal neurons from loss and increased local synaptic proteins. Compared to aged wildtype mice, aged STING knockout mice had reduced microglial accumulation and increased neuronal density in the hippocampus.
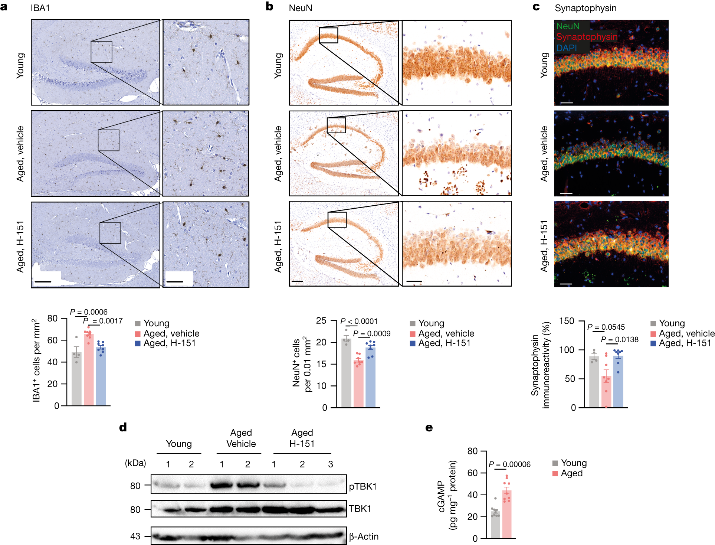
Analysis of whole brain homogenates from old mice showed increased levels of phosphorylated TBK1, responsible for STING downstream signaling. Acute STING inhibition in old mice reduced pTBK1 signaling, indicating the STING-TBK1 signaling axis participates locally in the aged brain.
In DNA sensing, STING is activated by 2'3'-cGAMP produced by cGAS. Strong cGAMP generation was detected in the brain homogenates of old but not young mice. The data indicated STING is activated in the brains of old mice, suggesting abnormal cGAS activity upstream of STING during aging.
Cellular functions of cGAS-STING signaling
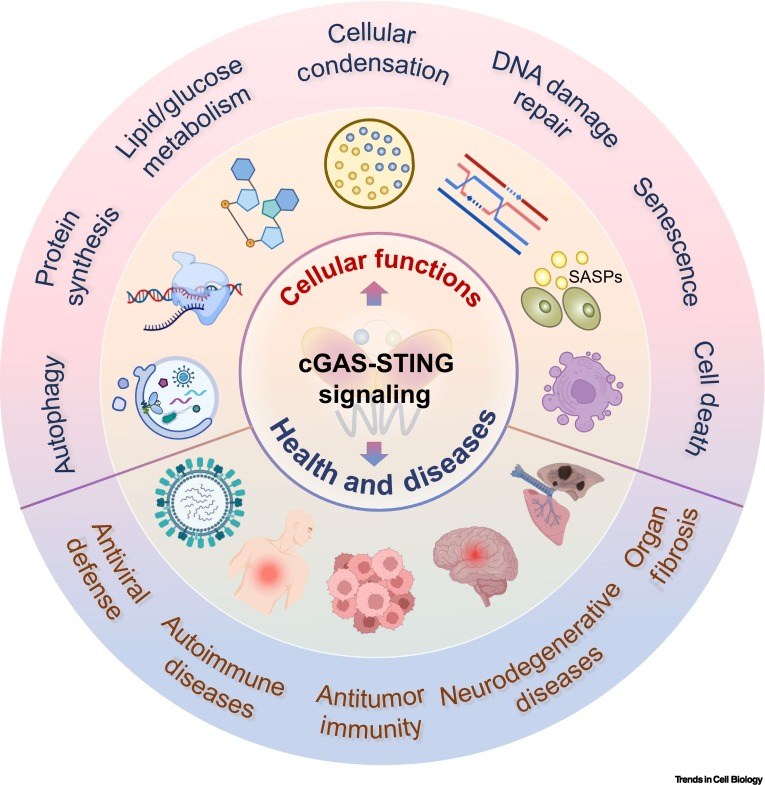
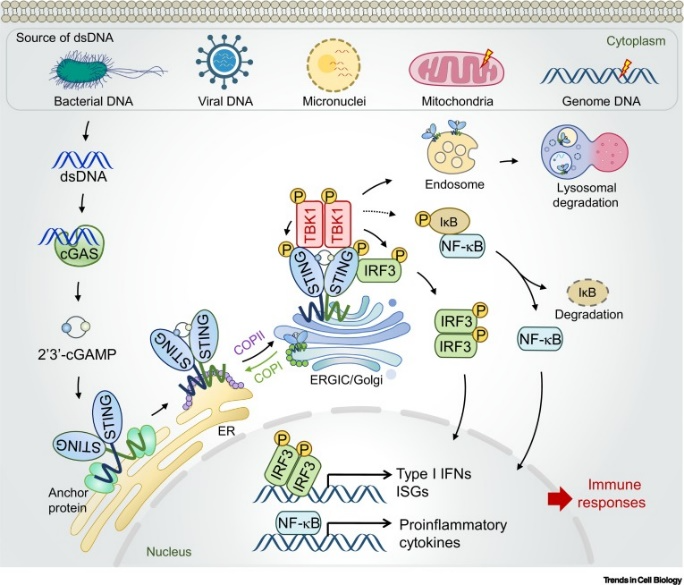
The cGAS-STING signaling pathway has increasingly garnered attention from researchers. The pathway has been demonstrated to play a crucial role in detecting misplaced genomic, mitochondrial, and microbial double-stranded DNA (dsDNA). When activated, the pathway synthesizes 2′3′-cGAMP, which mobilizes the stimulator of interferon genes (STING) protein to initiate innate immune responses against tissue damage and pathogen invasion.
While the cGAS-STING pathway is essential for immune surveillance, imbalances of this signaling have been implicated in various diseases, including infections, autoimmune disorders, cancer, fibrosis, and neurodegeneration. cGAS-STING signaling plays multifaceted roles at the cellular level, including autophagy, translation, metabolism homeostasis, cellular condensation, DNA damage repair, senescence, and cell death.
cGAS-STING signaling exerts its effects through both the canonical and noncanonical pathways. The canonical pathway involves the induction of type I interferons (IFNs) and an array of IFN-stimulated genes (ISGs), while the noncanonical functions encompass senescence, autophagy, immune cell death, and proliferation regulation. Additionally, a recently identified noncanonical pathway directly activates a signaling cascade in the endoplasmic reticulum, controlling mRNA translation and contributing to innate immunity.
Understanding how the cGAS-STING pathway triggers these diverse cellular processes remains a challenge, but it holds great potential for the development of therapeutic strategies. By exploiting the cellular functions and mechanisms of cGAS-STING signaling, researchers hope to identify promising targets for the treatment of various diseases.
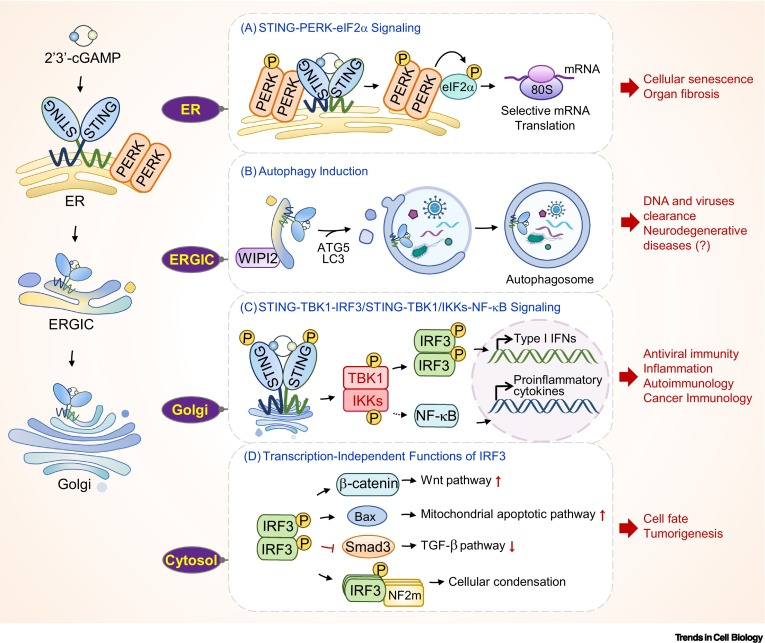
The multifaceted functions of cGAS-STING signaling are dependent on its ability to sense both microbial and host-derived DNAs, thereby shaping cellular processes and contributing to the progression of diverse pathological conditions. A deeper understanding of these processes will pave the way for the design of novel therapeutic approaches for degenerative diseases associated with the cGAS-STING pathway.
The studies represent significant advancement in our understanding of the cellular functions and disease relevance of cGAS-STING signaling, especially the inflammation associated with aging, and provided potential strategies to slow cognitive decline in age-related neurodegenerative diseases. Precisely elucidating neuroimmune crosstalk that controls microglia-dependent neurotoxicity also holds promise for future neurodegenerative disease research.
References
1.Gulen, M.F., Samson, N., Keller, A. et al. cGAS–STING drives ageing-related inflammation and neurodegeneration. Nature (2023).
2.Chen et al., Cellular functions of cGAS-STING signaling, Trends in Cell Biology, August 2023, Vol. 33, No. 8.