How do AMPK activators target the intestines?
Founded in 2015, Kallyope Inc. is a biotechnology company focused on the therapeutic potential of the gut-brain axis. Kallyope recently disclosed three patents pertaining to AMPK small molecule activators.
AMPK, which stands for AMP-activated protein kinase, is a key molecule in regulating energy metabolism and is at the core of research into diabetes and other metabolic-related diseases. AMPK is widely expressed in various metabolically related organs, and AMPK activators can potentially treat ailments such as allergies, cancers, depression, type 2 diabetes, malnutrition, obesity, psoriasis, and ulcerative colitis. However, the activation of AMPK through medication is challenging due to the potential existence of 12 different compound forms of AMPK in an animal's body.
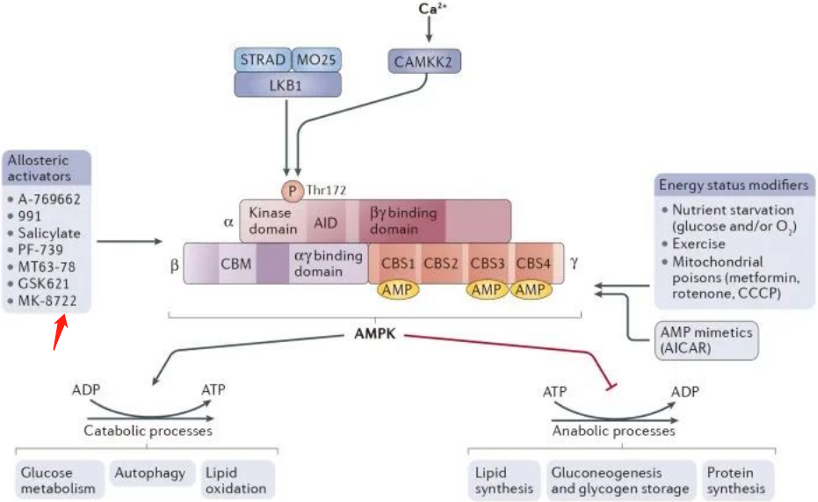
The AMPK mechanism is activated when the upstream kinase CAMKK2 is activated by intracellular calcium ions, and when LKB1 forms a heterotrimer with STRAD and MO25. Factors leading to the activation of AMPK include oxygen stress, glucose starvation, exercise, and mitochondrial toxins. Drugs that activate AMPK are primarily small molecule activators.
AMPK, a serine/threonine kinase, is evolutionarily conserved from yeast to mammals. As an energy sensor, AMPK's activity increases when the cellular ratio of 5'-adenosine monophosphate (AMP) to adenosine triphosphate (ATP) rises due to nutrient deprivation. Activated AMPK phosphorylates downstream substrates, promoting catabolic metabolism and inhibiting anabolic metabolism, leading to ATP production and energy recovery. The activity of AMPK can change due to various physiological factors (like hormones, cytokines, and dietary nutrients) as well as pathological conditions (like obesity, chronic inflammation, and type 2 diabetes). AMPK activation can lead to reduced hepatic glucose production and plasma glucose levels. Thus, AMPK is an attractive therapeutic target for various metabolic diseases.
In addition, AMPK has beneficial effects on intestinal health, such as enhancing intestinal absorption, improving barrier function, inhibiting the occurrence of colorectal cancer, reducing intestinal inflammation and metabolic-related diseases, and is very important for maintaining intestinal homeostasis. For instance, AMPK activation strengthens intercellular connections, nutrient transport, autophagy and cell apoptosis, suppressing intestinal inflammation and carcinogenesis. Hence, AMPK is related to the maintenance of tight connections of colonic epithelium, controlling the progression of colitis.
In various mouse models of colitis, the direct use of AMPK activators for treatment has been proven to effectively restore intestinal barrier function. Metformin is an indirect AMPK activator with additional biological activity. However, the persistent direct activation of AMPK raises safety concerns, particularly in the heart. Chronic treatment with systemic direct activators in rodents and non-human primates can lead to cardiac hypertrophy (with increased cardiac glycogen). Moreover, human genetic polymorphisms of AMPK are associated with cardiac glycogen deposition, cardiac hypertrophy, and Wolff-Parkinson-White syndrome. Due to the risk of cardiac hypertrophy, it is not suitable to directly use known AMPK activators for the treatment of IBD, colitis, and other intestinal barrier leakage diseases.
All the direct AMPK activators that have been reported into clinical studies (such as Pfizer's PF-06409577, which has been terminated in phase I) or broad preclinical evaluations (like Merck's MK-3903 and MK-8722) are systemic AMPK activators and have been developed for systemic administration. It has also been reported that a slow-release formulation, delivering a higher concentration of the indirect AMPK activator metformin to the colon for IBD treatment. However, metformin does not optimally activate AMPK, and metformin has other activities, thus this method requires specific formulation development. Therefore, it is not the optimal solution to the problem.
The patented compound disclosed by Kallyope Inc. is the first discovery and development of a gut-restricted direct AMPK activator that can reach the target tissue and avoid systemic circulation without the need for complex formulation.
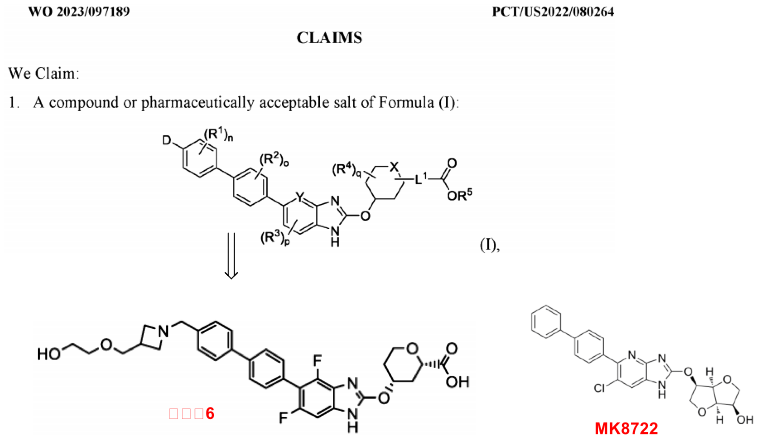
The general formula disclosed in patent WO2023097189 (as shown above) represents a class of Benzimidazole-type small molecule compounds with biphenyl substituents. The patent includes 60 embodiments, with Merck's MK-8722 as a reference for comparison. In ADP-Glo assays, the most potent compound, 6, activates recombinant AMPK (EC50 = 0.2 nM), outperforming MK8722’s EC50 = 4.7 nM by 23.5 times. Compound 6 is derived from MK8722 by substituting MK8722's isosorbide substituent with a pyranic acid substituent, then extending the biphenyl substituent of MK8722 to include a nitrogen heterocyclic ethylene glycol substituent. This modification strategy aims to concentrate the drug in the intestine, decreasing systemic distribution and thereby reducing oral bioavailability. The patent also includes research on the structure-activity relationship of changing the 4-membered nitrogen heterocyclic ring to a 5-membered one and aromatic heterocyclic rings, but only reveals in vitro activity data. MK-8722 as an AMPK activator can activate all 12 types of AMPK complexes. Merck reported the discovery process of MK8722 in a 2017 Science paper, which showed its regulatory effect on glucose metabolism and promising effect on diabetes in animal models, without causing hypoglycemia. In tests on rats and marmosets over 6-8 months at high doses of MK-8722, no hypoglycemia incidents were found. However, MK-8722 can induce myocardial hypertrophy, and ultimately, it did not enter clinical trials.
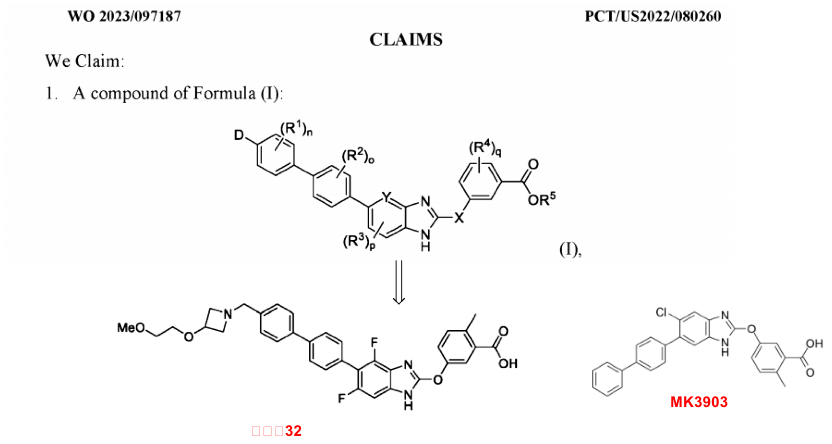
The general formula disclosed in patent WO2023097187, which also represents a class of Benzimidazole-type small molecule compounds with biphenyl substituents, includes 130 embodiments, with Merck's MK-8722 and MK3903 as references. The activities of most compounds in this patent exceed the previous one and uniquely, oral PK (pharmacokinetics) data are disclosed. In ADP-Glo assays, the most potent compounds, 30 and 32, activate recombinant AMPK (EC50 < 0.01 nM), compared to MK8722’s EC50 = 4.7 nM and MK3903’s EC50 = 20.9 nM. In C57BL/6 mice, oral bioavailability (F) is 4% (30 mg/kg p.o.), significantly reducing systemic distribution. Compound 32 is derived from MK3903 by substituting its chlorine benzene with difluorobenzene and extending the biphenyl substituent of MK3903 to include a nitrogen heterocyclic ethylene glycol methylester substituent. The patent also includes research on the structure-activity relationship of changing the 4-membered nitrogen heterocyclic ring to a 5-membered one, and aromatic heterocyclic rings. MK3903, also developed by Merck and currently preclinical, is reported to activate only 10 out of the 12 phosphorylated AMPK (pAMPK) complexes, with an EC50 value range from 8 to 40 nM, and max activation >50%. MK-3903 partially activates pAMPK5 (max 36%), but does not activate pAMPK6. Also, MK-3903 has low permeability and is a substrate for the human hepatic uptake transporter proteins OATP1B1 and OATP1B3. These shortcomings also led to Merck's decision not to advance it to clinical trials.
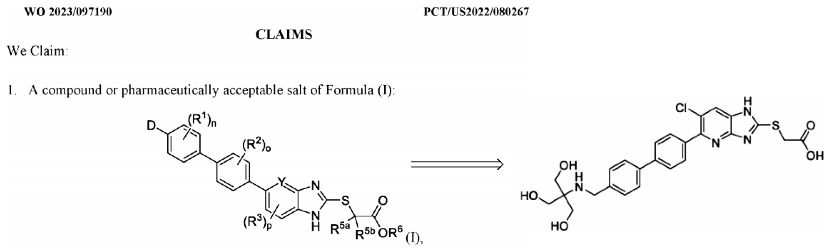
See the other patent in the figure below, which primarily involves introducing a sulfonated carboxylic acid segment into a benzimidazole moiety. However, the patent only contains 21 compounds, and the strength of the activity data is merely represented by letters. As shown in the figure, the introduction of three hydroxyethyl substituents and a sulfonated carboxylic acid substituent could potentially enhance intestinal targeting and decrease oral bioavailability.
In summary, Kallyope Inc. has disclosed three patents, completed over 200 implementation examples, and led in the discovery and development of MK-3903 and MK-8722 as gut-restricted, direct AMPK activators, which can reach target tissues and avoid systemic circulation without the need for complex formulations.