Cytokinetics Unveils Results From Initial Phase Study of CK-4021586
Cytokinetics, Incorporated (Nasdaq: CYTK) disclosed that data from the Phase 1 clinical trial of CK-4021586 (CK-586) were showcased during a poster presentation at the American College of Clinical Pharmacology (ACCP) Annual Meeting, held in Bethesda, MD. The trial successfully achieved its primary and secondary goals of evaluating the safety, tolerability, and pharmacokinetics (PK) of single and repeated oral doses of CK-586. These findings endorse the progression of CK-586 to a Phase 2 clinical study in patients suffering from heart failure with preserved ejection fraction (HFpEF), anticipated to commence in the fourth quarter of 2024. CK-586 is a cardiac myosin inhibitor being developed for potentially treating a specific group of HFpEF patients characterized by hypercontractility and ventricular hypertrophy.
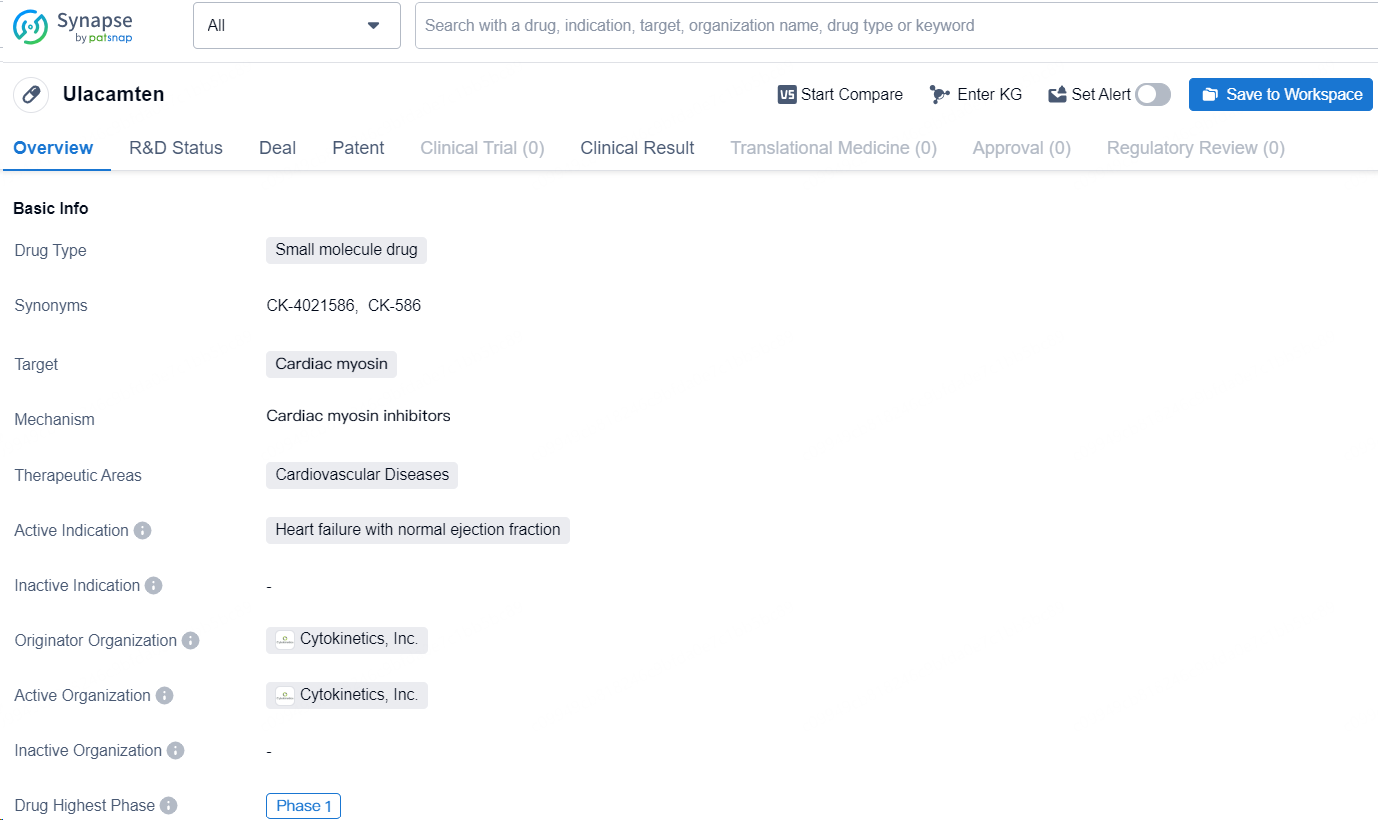
👇Discover comprehensive information about this drug, from its R&D status, core patents, clinical trials to approval status in global countries, by simply clicking on the image below. Dive deep into our drug database now.
"The findings from this Phase 1 trial confirm pre-clinical data indicating that CK-586 directly diminishes cardiac contractility at the sarcomere level. Notably, CK-586 exhibited a shallow and predictable pharmacokinetic/pharmacodynamic (PK/PD) relationship and a half-life that supports a once-daily fixed dosing regimen for patients with heart failure with preserved ejection fraction (HFpEF)," stated Stuart Kupfer, M.D., Senior Vice President, Chief Medical Officer. "We are preparing for a Phase 2 clinical trial of CK-586 in a specific subgroup of HFpEF patients, which we aim to commence in the fourth quarter."
Phase 1 Study Design and Principal Findings
The primary aim of this Phase 1 double-blind, randomized, placebo-controlled, single and multiple ascending dose study was to assess the safety, tolerability, and pharmacokinetics (PK) of orally administered CK-586 in healthy volunteers. The study incorporated seven single ascending dose groups (10 mg to 600 mg), each with 10 participants, and two multiple dose groups (100 mg and 200 mg daily), also with 10 participants in each.
The study data revealed that CK-586 was safe and well-tolerated among healthy participants. No severe adverse events were reported, and the study’s stopping criteria were not triggered. The observed half-life of CK-586 ranged from 14 to 17 hours. CK-586 showed dose-linearity across a broad range of exposures without altering the half-life, achieving steady state within seven days of dosing. Left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) decreased from baseline in a manner proportional to exposure, demonstrating a shallow and predictable PK/PD relationship (Figure 1). At the highest single dose of 600 mg, the average reduction in LVEF was less than 5%. These outcomes illustrate pharmacologic traits that might support once-daily fixed-dose administration in future treatments. Plans for a Phase 2 clinical trial in HFpEF patients are ongoing, with the trial slated to begin in Q4 2024.
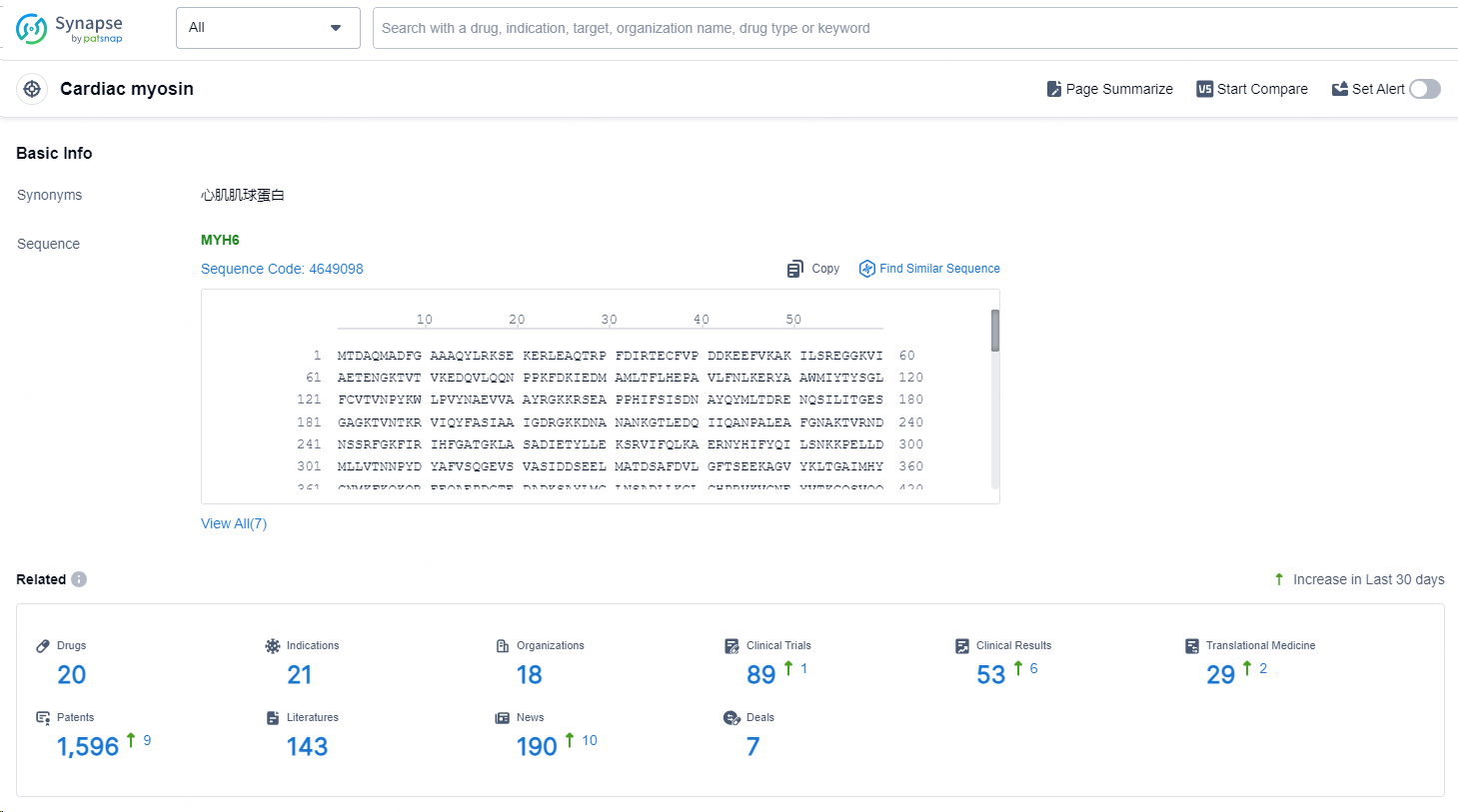
👇Explore the latest research progress on drug-related developments, indications, therapeutic organizations, clinical trials, results, and patents by clicking on the targeted picture link below. Unfold a world of comprehensive information on this target in just a click!
According to the data provided by the Synapse Database, As of September 14, 2024, there are 20 investigational drugs for the cardiac myosin targets, including 21 indications, 18 R&D institutions involved, with related clinical trials reaching 89, and as many as 1596 patents.
The drug Ulacamten is a small molecule drug developed by Cytokinetics, Inc. It targets cardiac myosin and is intended for the treatment of cardiovascular diseases, specifically heart failure with normal ejection fraction. As of the latest available information, Ulacamten has reached the highest phase of Phase 1 clinical trials globally.