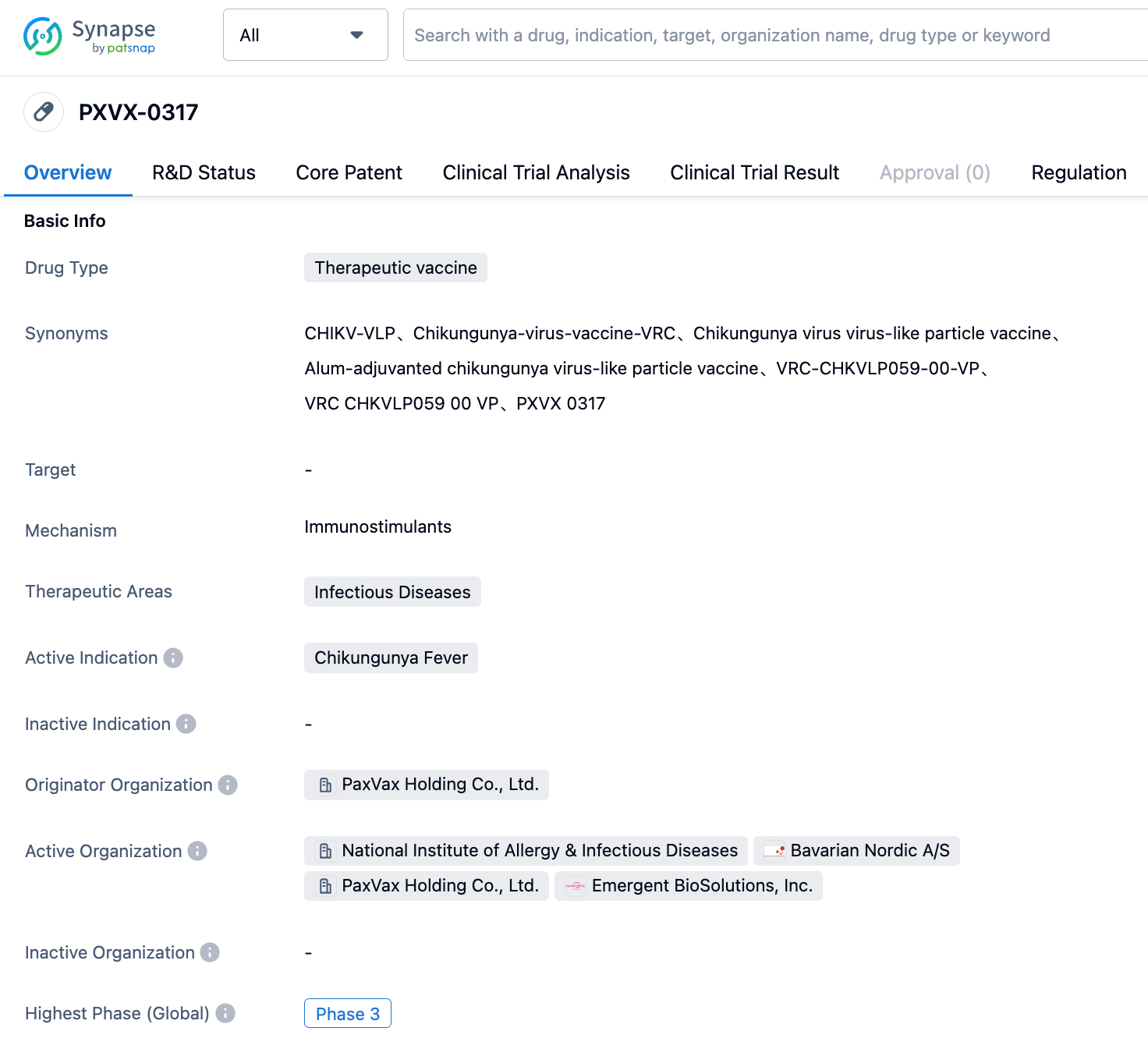
Positive Results of Phase III Clinical Trials for Bavarian's Chikungunya Virus Vaccine PXVX0317
On August 8, 2023, Bavarian Nordic announced positive top-line results from a randomized, double-blind, placebo-controlled phase 3 clinical trial of its Chikungunya virus candidate vaccine, CHIKV VLP (PXVX0317), in adults and adolescents aged 12 to 64. The Chikungunya candidate vaccine met all the common main endpoints of this phase 3 trial and showed high immunogenicity in the majority of participants after a single dose on day 22. The company plans to submit regulatory applications in 2024.
PXVX0317 is an adjuvant virus-like particle (VLP) based investigational vaccine for active immunization against Chikungunya. The drug has received breakthrough therapy designation and fast track status from the US FDA, and PRIME status from the European Medicines Agency (EMA). Chikungunya fever is a mosquito-borne viral disease caused by the Chikungunya virus (CHIKV). CHIKV disease usually presents with acute symptoms, including fever, rash, fatigue, headache, and severe and debilitating joint pain. Although mortality is very low, morbidity is high; nearly 50% of CHIKV patients have long-term debilitating symptoms, which can increase with age. In the past 20 years, CHIKV has emerged in several previously non-endemic regions in Asia, Africa, Southern Europe, and the Americas, often leading to unpredictable large-scale outbreaks. There is currently no effective treatment or vaccine.
The announced Phase 3 trial enrolled a total of 3,254 participants who were randomized to receive a single intramuscular injection of CHIKV VLP or placebo. Results from Day 22 post-vaccination showed that CHIKV VLP had high immunogenicity in healthy adolescents and adults, inducing strong Chikungunya neutralizing antibodies in 98% of vaccine recipients. Strong neutralizing antibody titers equal to or exceeding the threshold set by regulatory agencies as serum protection markers were achieved, meeting the primary endpoint of the trial. In addition, CHIKV VLP induced significant neutralizing antibodies in 97% of vaccinees within two weeks after vaccination, confirming the vaccine's ability to quickly trigger protective immunity levels. The response generated was stable and had longevity, with 86% of participants still having neutralizing antibody levels that reached serum protection levels 6 months post-vaccination.
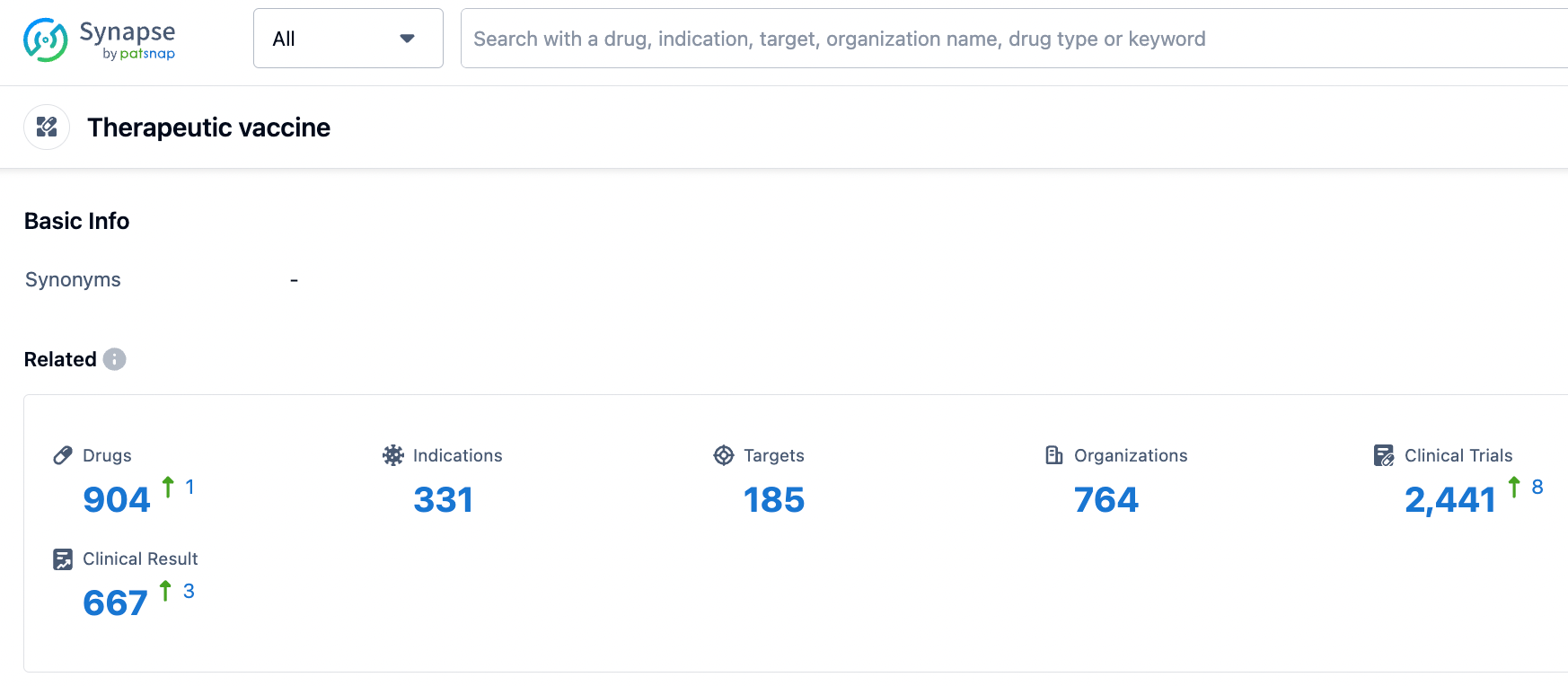
According to information disclosed by Synapse, as of August 9, 2023, there were 904 investigational drugs in the therapeutic vaccine category, covering 331 indications, 185 targets, 764 research institutions, and 2,442 related clinical trials......Looking forward to PXVX0317 getting approved soon to provide new protective options for people.