Expanding the Horizons of CAR T Therapy Beyond Cancer Treatment: Part II
With the rapid progress of gene editing technology and mRNA vaccine technology, as well as the continuous exploration of researchers, CAR-T therapy is actively fused with various emerging technologies in an attempt to overcome the challenges it faces.
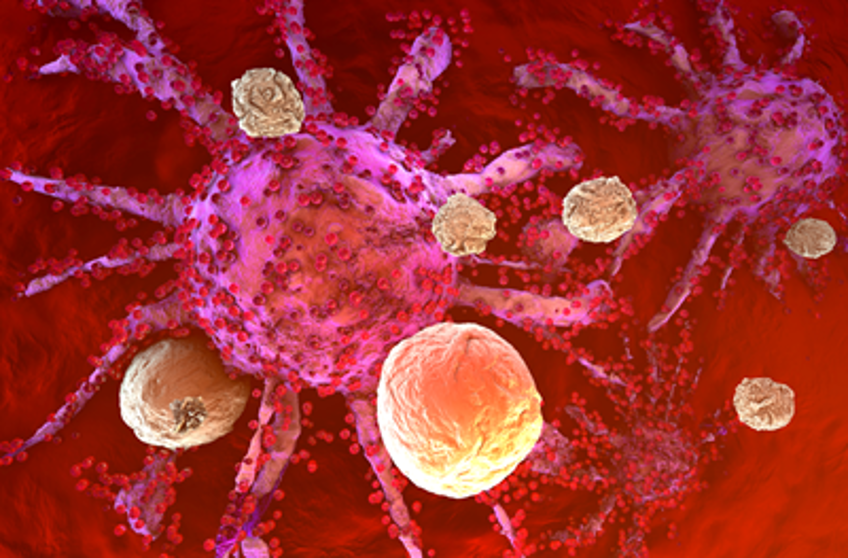
mRNA targeted delivery technology
The approval of mRNA vaccines for COVID-19 by the FDA has demonstrated the broad effectiveness of mRNA therapy. Although the currently approved therapies are non-targeted, several clinical trials are underway using targeted lipid nanoparticles (tLNP) to deliver mRNA specifically to selected cells. This technology is primarily achieved by conjugating targeted antibodies to the surface of LNPs.
Various research groups have demonstrated that modified mRNA CAR can be delivered to T cells in vivo. In leukemia and prostate cancer mouse models, CAR-T cells engineered with mRNA performed comparably to CAR-T cells engineered with retroviruses, effectively controlling tumors and increasing survival rates. Another study showed that tLNP can deliver anti-FAP CAR mRNA to T cells in mice, resulting in CAR expression and successfully reducing fibrosis, ultimately improving cardiac function in a heart failure model, similar to the results obtained with anti-FAP CAR-T cells engineered with retroviruses. Therefore, a single injection of tLNP-encapsulated mRNA can generate functional and therapeutically relevant CAR-T cells in vivo.
Compared to current clinical methods, tLNP-mediated CAR mRNA delivery has several key advantages. First, cost. Currently, CAR-T therapy requires an expensive ex vivo manufacturing process. Generating CAR-T cells in vivo will potentially reduce production costs and enable faster scale-up.
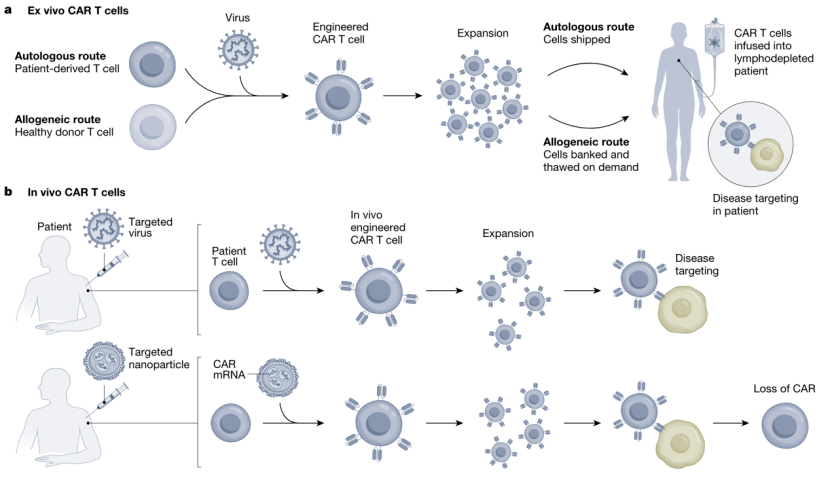
Second, safety. The current therapy requires lymphocyte depletion before CAR-T cell infusion to optimize engraftment, a process that increases the risk of cytokine release syndrome due to acute CAR-T expansion and the risk of secondary malignancies due to genotoxicity. In vivo generation could potentially avoid these issues and improve safety.
The most important potential advantage is transient expression. mRNA delivery causes T cells to express CAR for a limited time, after which expression is lost as the mRNA degrades. In contrast, current CAR-T cells stably express CAR and can persist in some patients for decades. While persistence is advantageous for cancer treatment, long-term CAR expression may be unnecessary or undesirable for other conditions. For example, one-time removal of cardiac fibrosis may suffice to improve heart function, while persistent inhibition of fibrosis could interfere with essential processes like wound healing. Of course, the safety of in vivo mRNA CAR-T still needs further study.
Viral vector-mediated in vivo delivery
In addition to targeted mRNA delivery, viral vectors can also be used to achieve in vivo CAR delivery, though this may still be more expensive than mRNA delivery. However, it would likely be more cost-effective compared to conventional CAR-T therapies.
However, this method carries the risk of off-target delivery, which could cause CAR delivery and integration into the genomes of unintended cells. If the viral vectors enter germ cells, it could result in serious transgenerational spread. The advantage of this method over mRNA is that it can produce long-lasting CAR-T cells, which is more suitable for diseases that require sustained CAR-T cells, such as cancer.
New technologies like mRNA delivery and gene editing are improving CAR-T therapy by enhancing efficacy, safety, and persistence. They hold great promise to help CAR-T therapy overcome current challenges and expand its applications.
Hurdles to overcome
The application of CAR-T in different disease domains and the results integrating emerging technologies have been verified in preclinical or early clinical stages, but there is still a long way to go for clinical translation and becoming an established therapy.
Firstly, it’s critical to find a suitable antigen, for which, the current method is to identify antigens that are specifically upregulated in target organs or cells through omics data. Identifying a targetable antigen is just the first step, safety issues still need to be addressed. Cytokine release syndrome (CRS) is the most common toxicity encountered clinically with CAR-T. Preclinical studies should repeatedly confirm whether CRS-like symptoms occur when applying new diseases.
In addition to CRS, CAR-T's clearance of physiological cells and potential side effects require attention. Eliminating healthy cell subsets (such as B cells in SLE) has a therapeutic effect. However, the long-term impact of clearing these cells is still unclear. As trials expand, we will understand whether clearance of healthy cells has long-term complications.
In addition, CAR-T clinical treatment has many unresolved issues:
1) Determining the optimal dose to balance efficacy and safety
2) Identifying the best time for patient administration
3) Establishing whether treatment should be terminated at some point
4) Exploring if removing pathological cells can restore healthy tissue vitality
5) Investigating CAR-T cell transport in non-cancerous tissues
Although there are still open questions, the progress continues. With more CAR-T products treating solid tumors, there is an increasing amount of long-term follow-up data available. Through reviewing past decades of research, we can draw valuable experience. The success of CAR-T in hematologic malignancies lays the foundation for treating solid tumors, and oncology results will provide crucial knowledges for converting CAR-T to other diseases.

Reference
Baker DJ, Arany Z, Baur JA, Epstein JA, June CH. CAR T therapy beyond cancer: the evolution of a living drug. Nature. Jul 2023;619(7971):707-715.