Decoding Durvalumab: a comprehensive study of its R&D trends and its clinical results in 2023 AACR
The latest clinical result of durvalumab + chemotherapy followed by adjuvant durvalumab in patients with resectable NSCLC was reported at the AACR Congress, demonstrating the clinical benefit of immunotherapy in either the neoadjuvant or adjuvant resectable (R) NSCLC setting.
Durvalumab's R&D Progress
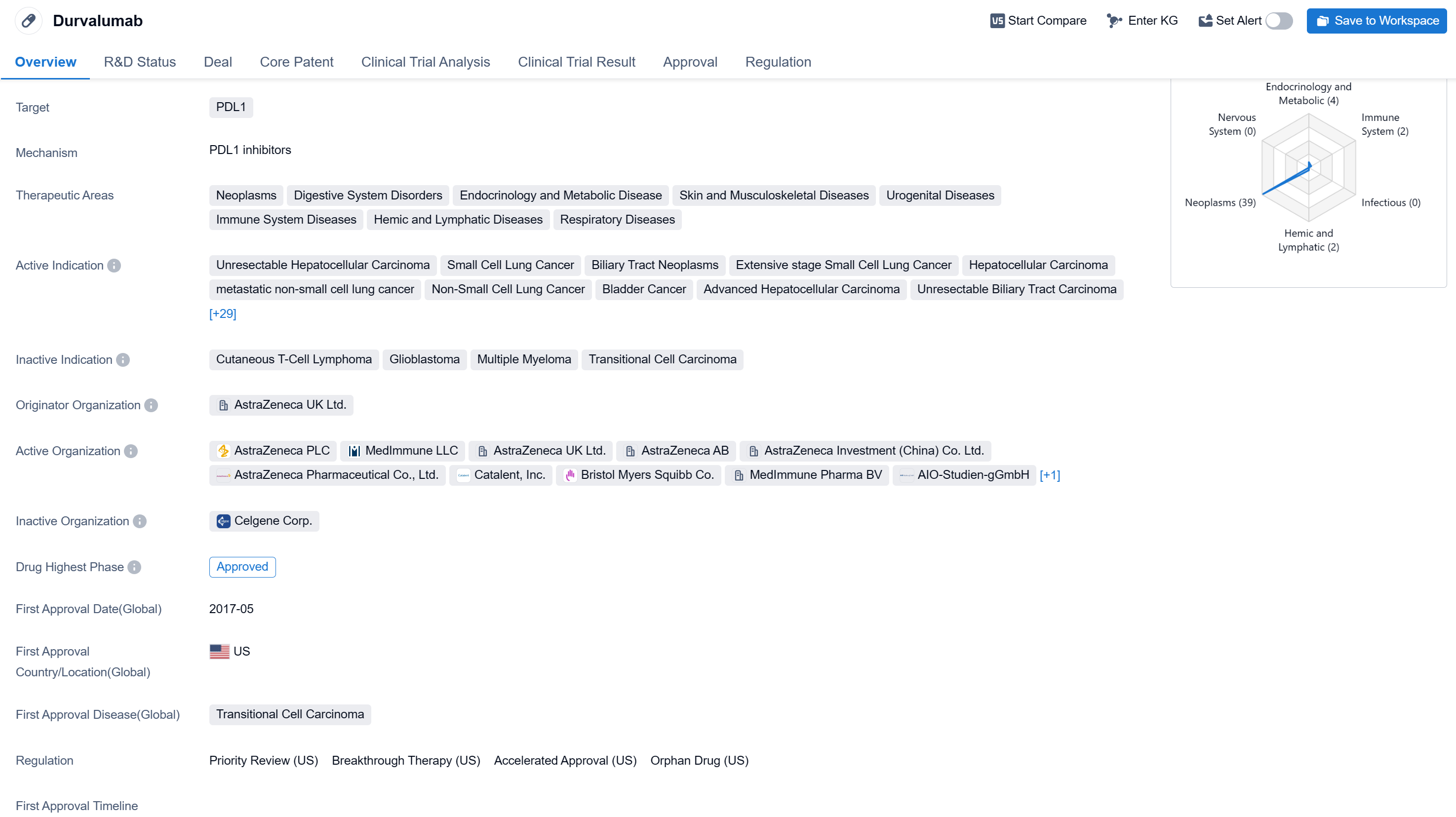
Durvalumab is a monoclonal antibody drug that targets PDL1, a protein involved in regulating the immune system. It has been approved for use in various therapeutic areas, including neoplasms, digestive system disorders, endocrinology and metabolic disease, skin and musculoskeletal diseases, urogenital diseases, immune system diseases, hemic and lymphatic diseases, and respiratory diseases.
According to the Patsnap Synapse, Durvalumab has received priority review, accelerated approval, orphan drug designation, and breakthrough therapy designation. And the clinical trial areas for Durvalumab are primarily in the United States, China, and United Kingdom. The key indication is Neoplasms. 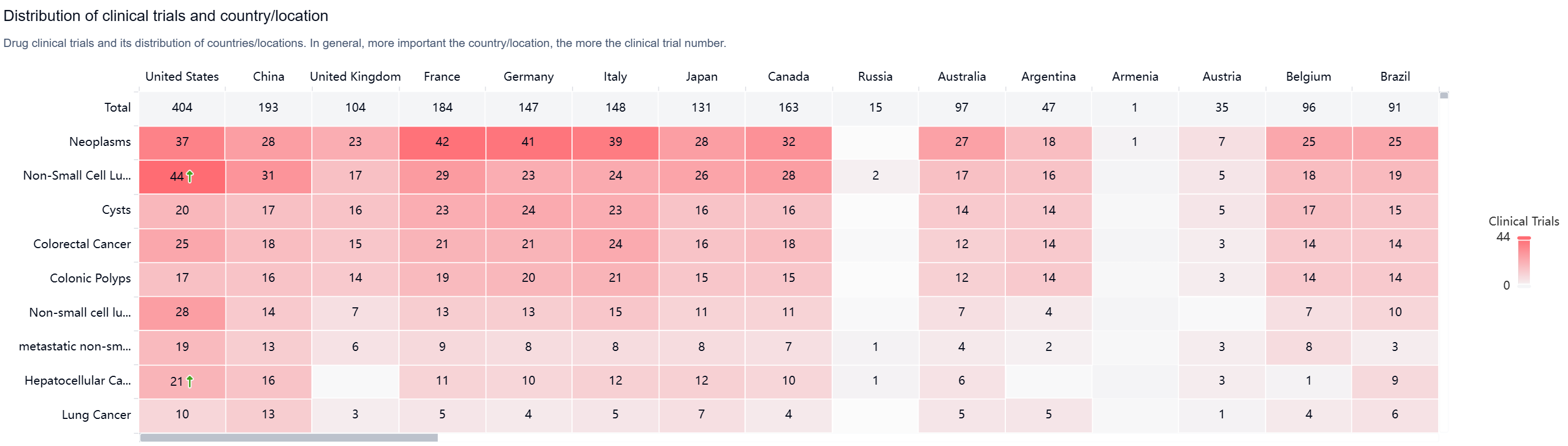
Detailed Clinical Result of Durvalumab
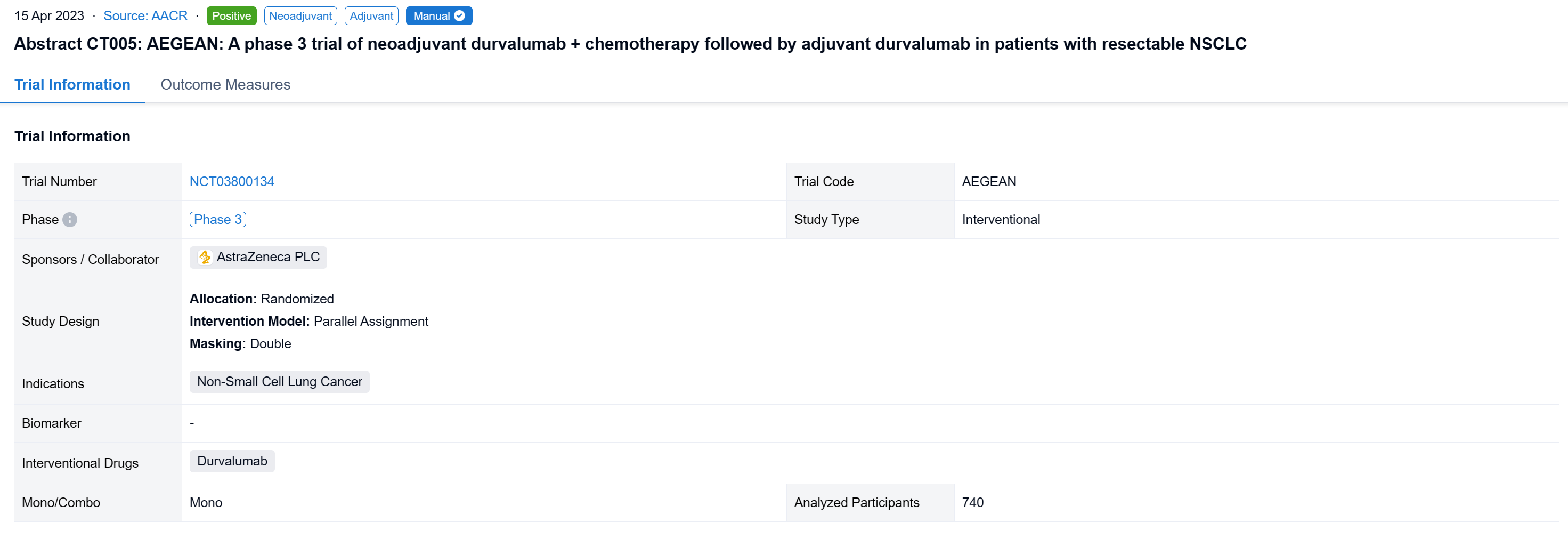
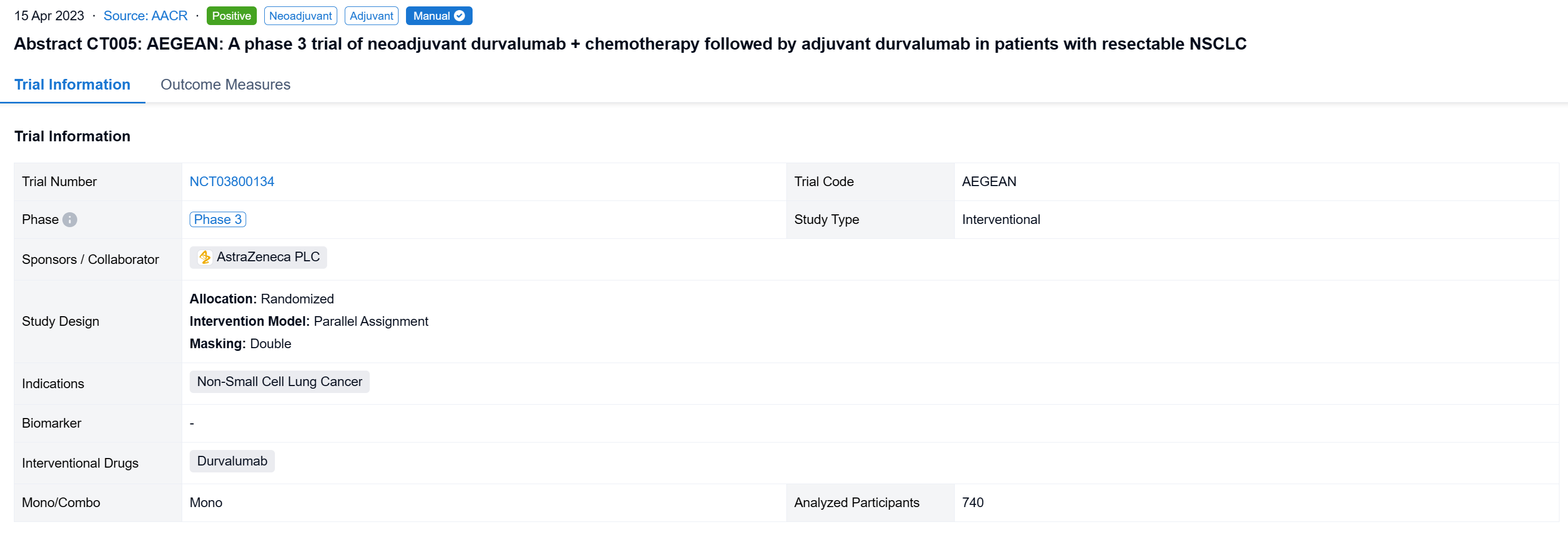
AEGEAN (NCT03800134) is a randomized, double-blind, placebo (PBO)-controlled trial assessing neoadjuvant durvalumab (D) + chemotherapy (CT) followed by surgery (Sx) and adjuvant D in patients (pts) with R-NSCLC.
In this study, adults with treatment (Tx)-naïve R-NSCLC (stage II-IIIB[N2]; AJCC 8th ed) and ECOG PS 0/1 were randomized (1:1) to receive D 1500 mg or PBO IV + platinum-based CT (every 3 weeks [Q3W] for 4 cycles) before Sx, then further D 1500 mg or PBO IV (Q4W, up to 12 cycles). Pts were stratified by disease stage (II vs III) and PD-L1 tumor cell expression (<1% vs ≥1%, Ventana SP263). Pts with documented EGFR/ALK aberrations were excluded from the modified intent-to-treat (mITT) population for efficacy analyses. The primary endpoints were pathological complete response (pCR), evaluated centrally, and event-free survival (EFS; using RECIST v1.1), evaluated by BICR. Safety was assessed in all pts who received ≥1 study Tx dose.
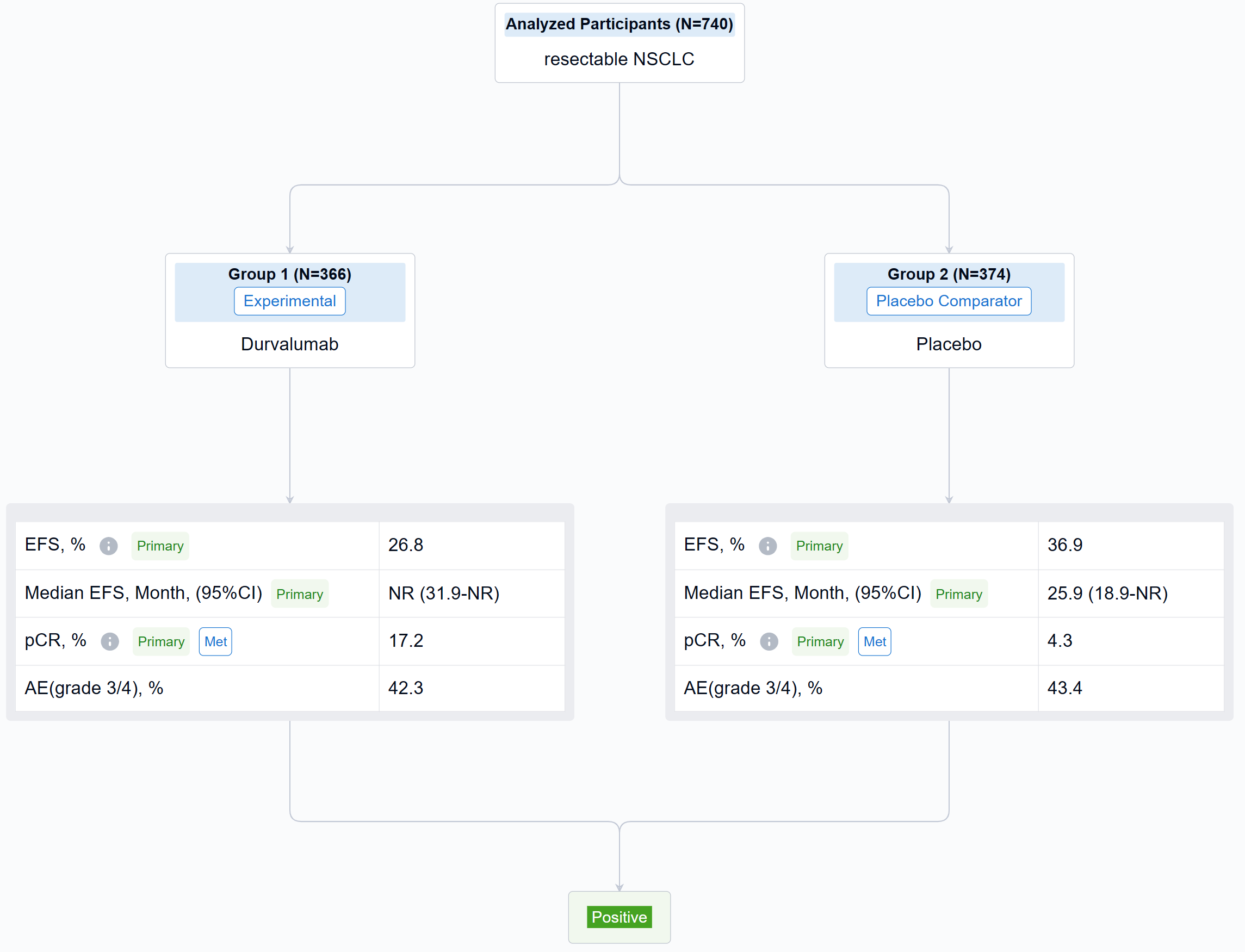
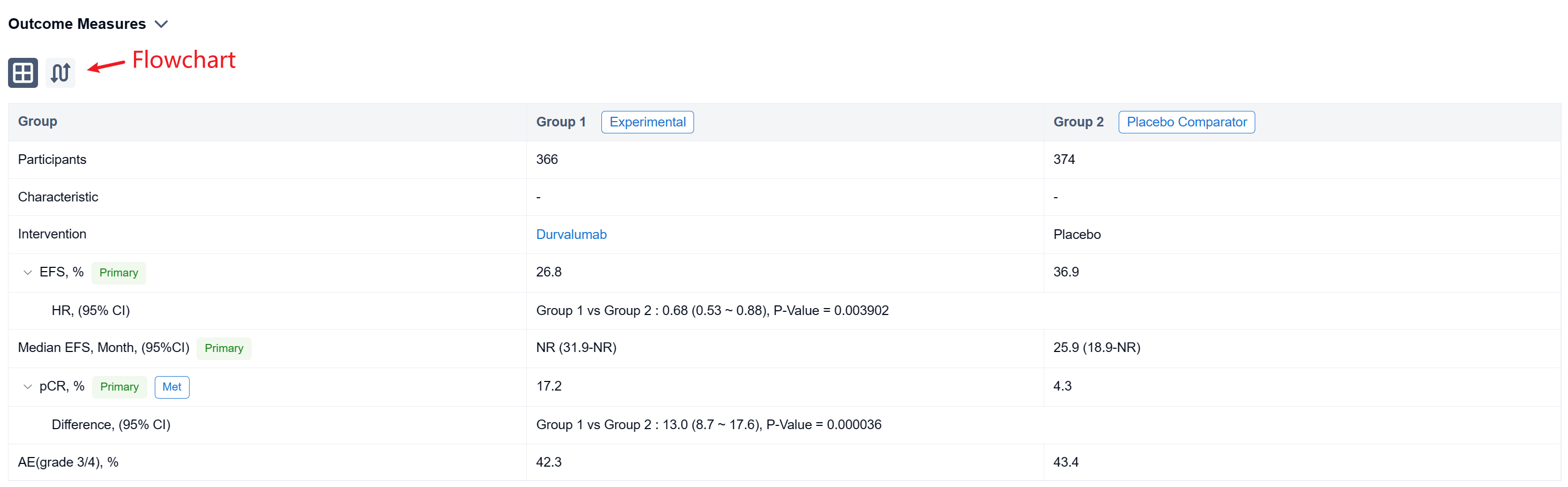
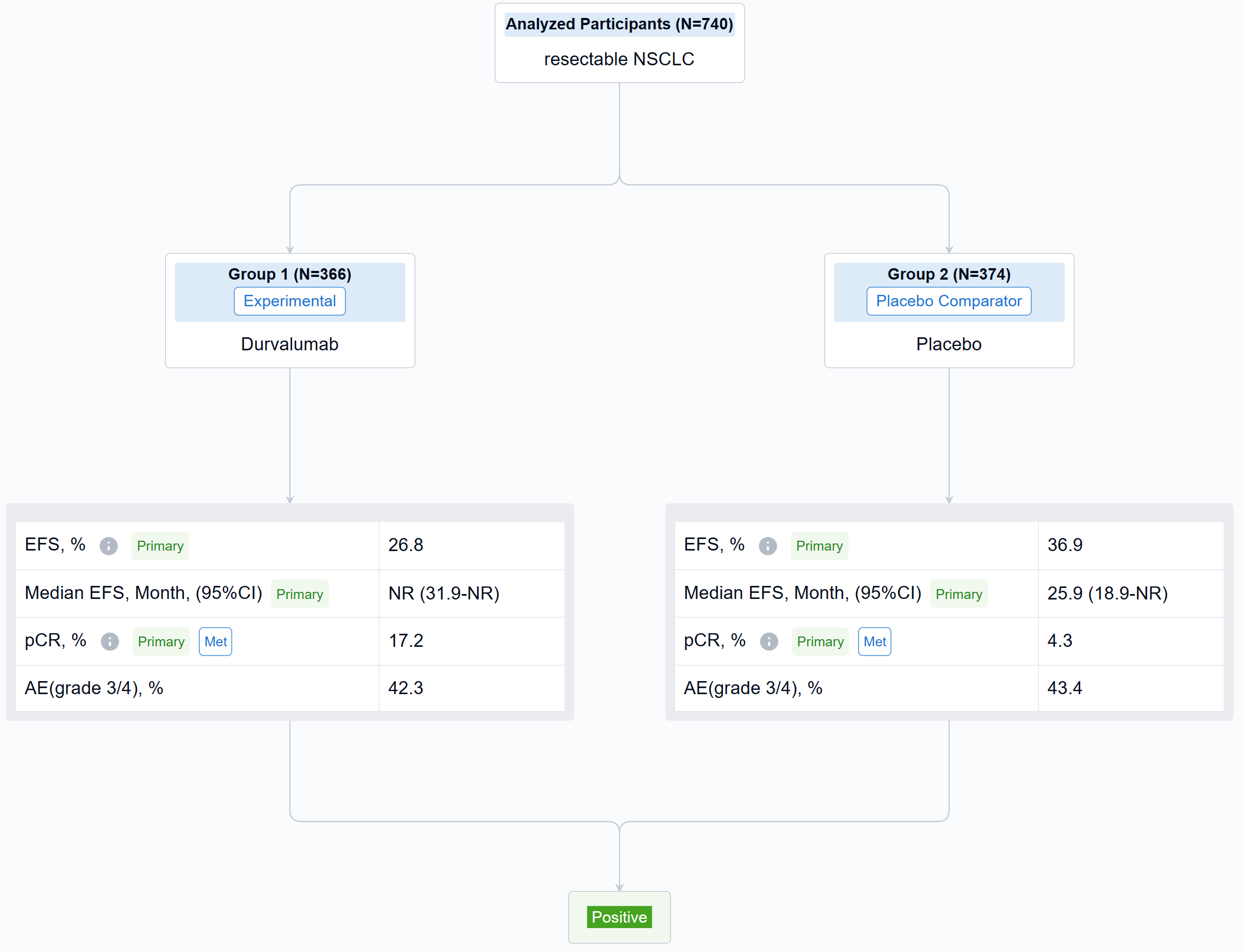
The result showed that between Jan 2, 2019, and Apr 19, 2022, 802 pts were randomized to the ITT population (n=740 in the mITT population) of whom 799 received Tx (D arm, n=400; PBO arm, n=399). Baseline characteristics were largely balanced (mITT). Overall, 84.7% in the D arm and 87.2% in PBO arm completed 4 cycles of platinum-doublet CT and 77.6% and 76.7%, respectively, completed Sx (mITT). As of Nov 10, 2022 (data cutoff), median EFS follow-up in censored pts was 11.7 months (mITT). The pCR rate was significantly higher and EFS significantly prolonged in the D vs PBO arms (mITT) (Table). In the safety analysis set, maximum grade 3/4 any-cause AEs occurred in 42.3% vs 43.4% in the D and PBO arms, respectively, during the overall Tx period.
It can be concluded that the AEGEAN trial met both of its primary endpoints of improved pCR and EFS. Perioperative D plus neoadjuvant CT was associated with a manageable safety profile.
How to Easily View the Clinical Results Using Synapse Database?
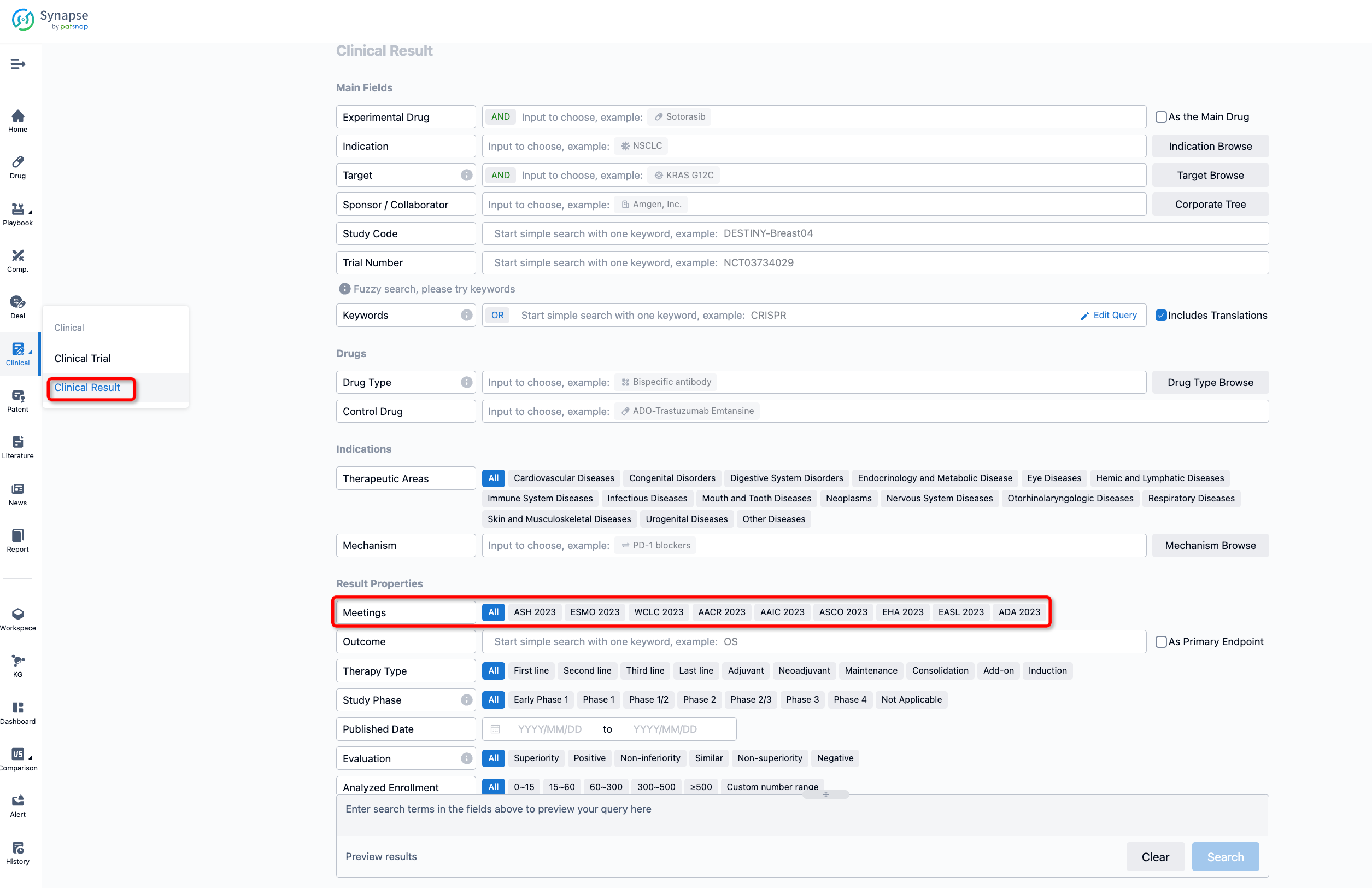
If you want to know the other clinical results of popular conferences, please lick on the “Clinical Results” on the homepage of Patsnap Synapse, which provides multi-dimensional screening and filtering of drugs, indications, targets, companies, result evaluation, release date, popular conferences, etc. to help you quickly locate the data you need.
Select the clinical meeting you are interested in, such as ESMO. In the results, you can quickly locate the data you want to view by indication, phase and drug name.
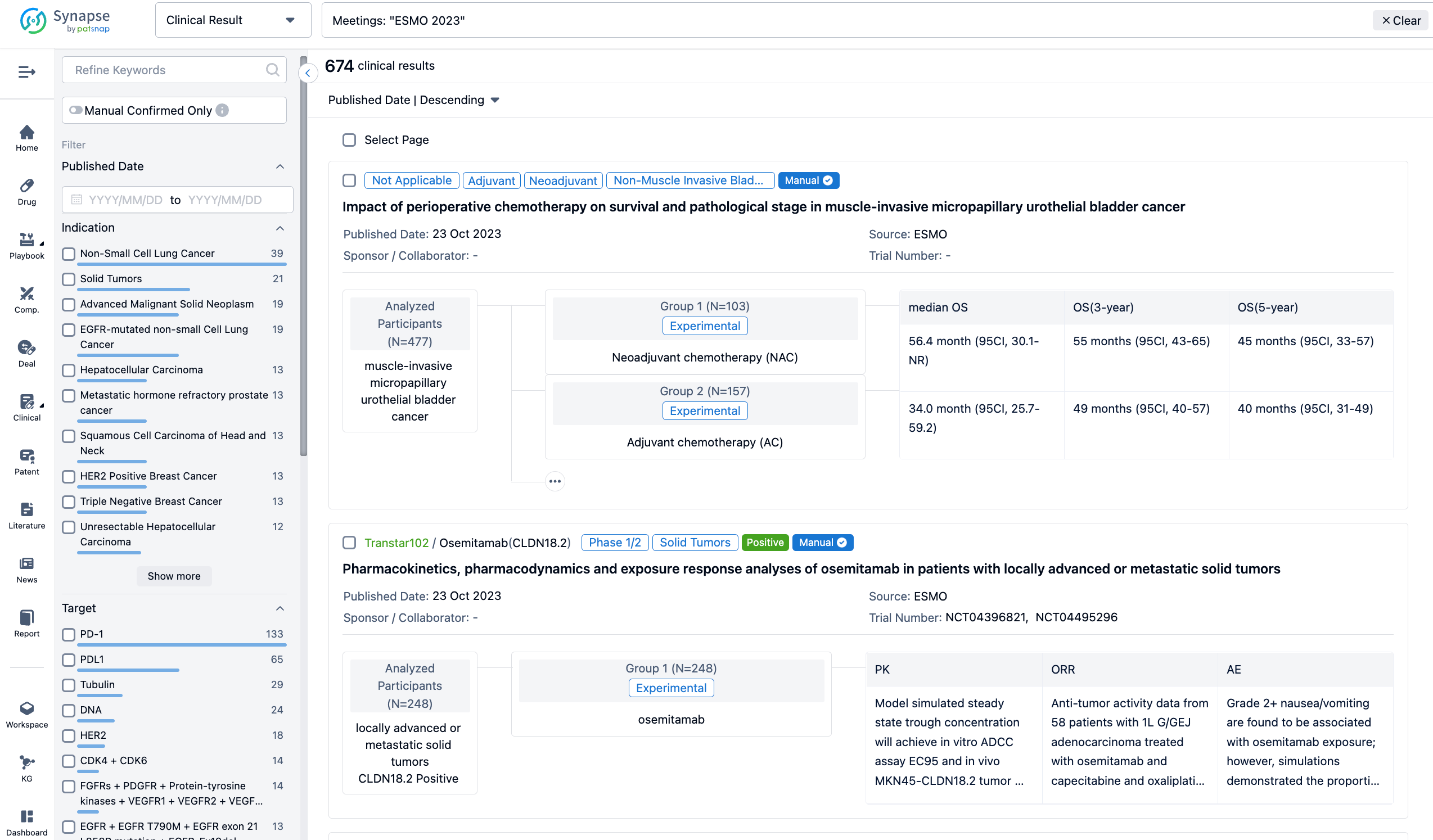
A single result clearly shows important information such as registration number, phase, indication, Sponsor/Collaborator, biomarker, Trial number, dosing regimen and more.
If you would like to view more information about this result, you can go to the result detail page by clicking on the title.
Above the headings, we provide the original source of the outcome data. The basic information is supplemented with more information beyond the list, such as company, study. design, etc.
In the important Outcome Measures section, we provide both list and flowchart forms, which are convenient for you to overview the comparison group information and core indicator data.
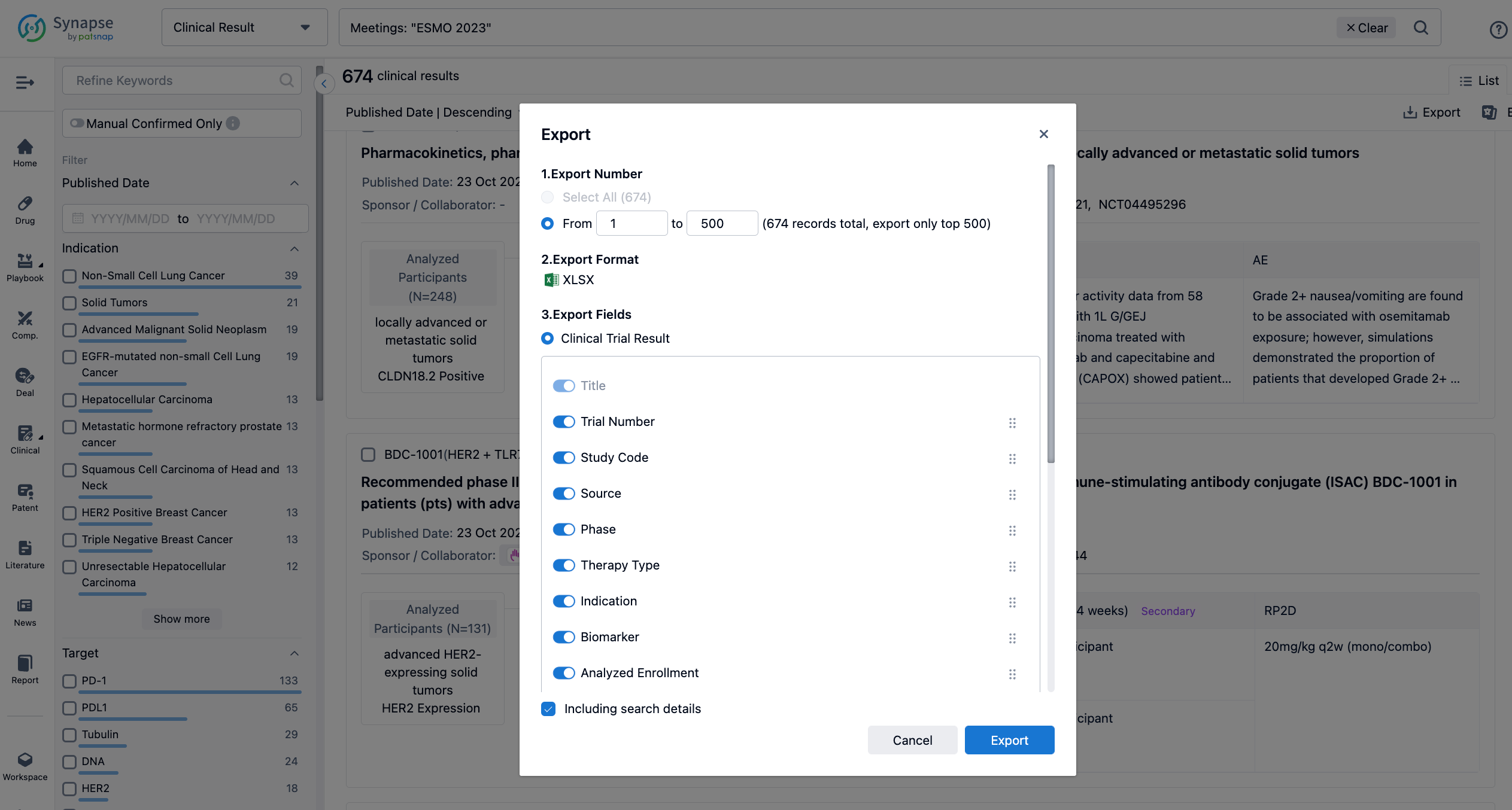
Finally, if you need to download these results, you can conveniently check the check boxes on the left side of the list, or directly click the "Export" button to download the data for personalized analysis and file sharing.
Click on the image below to embark on a brand new journey of drug discovery!