Decoding efalizumab: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Efalizumab's R&D Progress
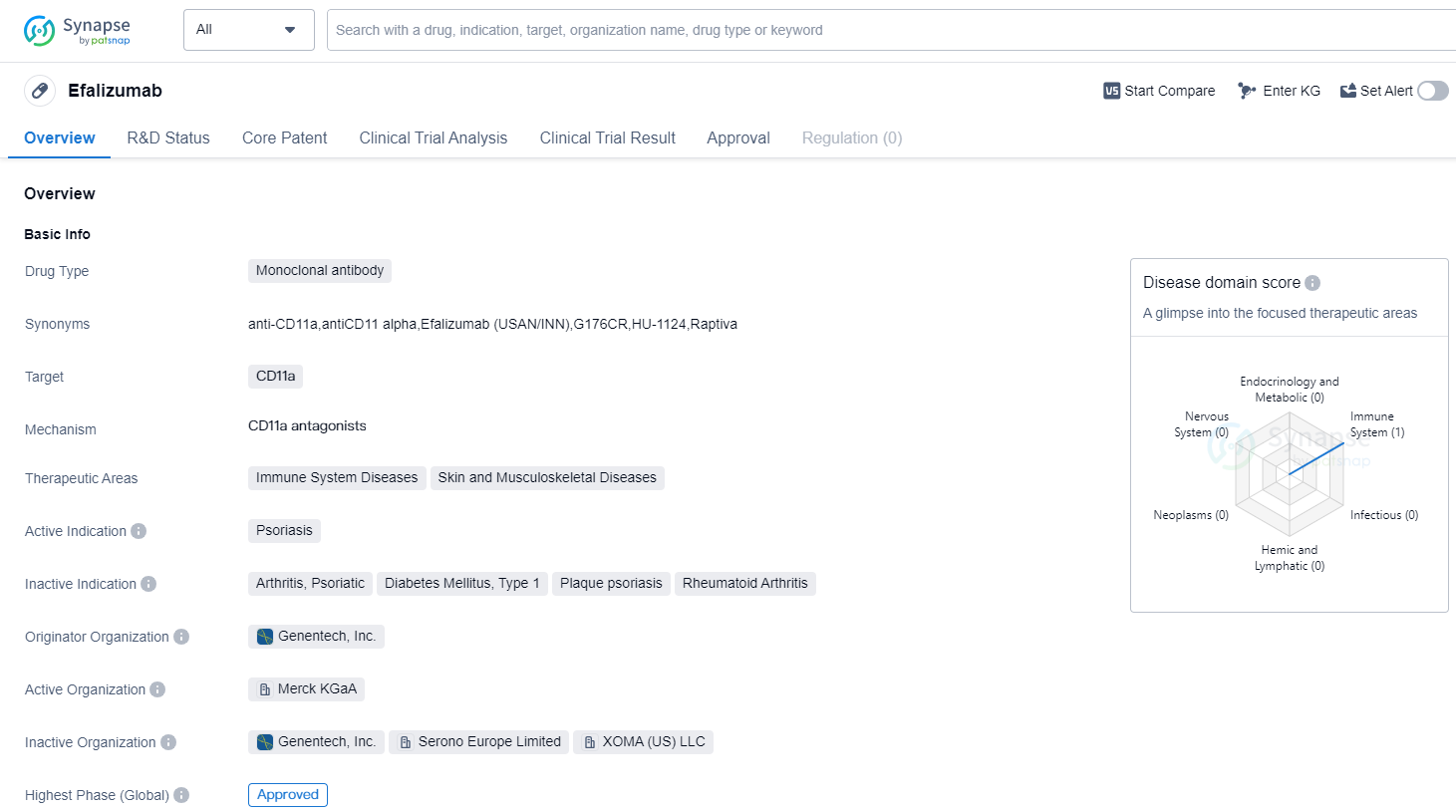
Efalizumab is a monoclonal antibody drug that targets CD11a and is primarily used for the treatment of psoriasis. It falls under the therapeutic areas of immune system diseases, as well as skin and musculoskeletal diseases. The highest R&D phase of this drug is approved.
Efalizumab is developed by Genentech, Inc., a renowned originator organization in the pharmaceutical industry. As a monoclonal antibody, it specifically targets CD11a, a protein involved in the immune system response. By targeting CD11a, efalizumab aims to modulate the immune system and alleviate the symptoms of psoriasis.
Psoriasis is a chronic autoimmune disease characterized by red, scaly patches on the skin. It is caused by an overactive immune system, leading to an excessive production of skin cells. Efalizumab works by inhibiting the activation and migration of T-cells, which play a crucial role in the immune response. By doing so, it helps to reduce the inflammation and abnormal cell growth associated with psoriasis.
The approval of efalizumab in 2003 marked a significant milestone in the treatment of psoriasis. Prior to its approval, treatment options for psoriasis were limited and often involved the use of topical creams or systemic medications with potential side effects. Efalizumab provided a targeted and more effective approach to managing the symptoms of psoriasis, offering relief to patients who had previously struggled to find adequate treatment.
Since its approval, efalizumab has been widely used in the United States and globally for the treatment of psoriasis. Its success in clinical trials and subsequent approval has paved the way for the development of other monoclonal antibody drugs targeting different aspects of the immune system. These advancements have revolutionized the treatment landscape for immune system diseases, including psoriasis, and have provided new hope for patients seeking effective and safe therapies.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for efalizumab: CD11a antagonists
CD11a antagonists are a type of drugs that target and inhibit the activity of CD11a, which is a cell surface protein involved in immune responses. CD11a is a subunit of the integrin receptor known as lymphocyte function-associated antigen 1 (LFA-1), which is primarily found on the surface of leukocytes, including T cells, B cells, and natural killer cells.
From a biomedical perspective, CD11a antagonists can be used to modulate immune responses in various diseases, particularly those involving excessive or inappropriate immune activation. By blocking the interaction between CD11a and its ligands, these antagonists can inhibit leukocyte adhesion and migration, thereby reducing inflammation and immune cell-mediated damage.
CD11a antagonists have shown potential in the treatment of autoimmune diseases, such as rheumatoid arthritis, psoriasis, and multiple sclerosis. By suppressing the immune response, these drugs can help alleviate symptoms and slow down disease progression. Additionally, CD11a antagonists have also been investigated as potential therapies for transplant rejection and certain types of cancer.
It's important to note that CD11a antagonists are a specific class of drugs that target CD11a. There are other types of antagonists that target different cell surface proteins or receptors involved in immune responses. The development and use of CD11a antagonists require careful consideration of their efficacy, safety, and potential side effects, as well as individual patient characteristics and disease context.
Drug Target R&D Trends for efalizumab
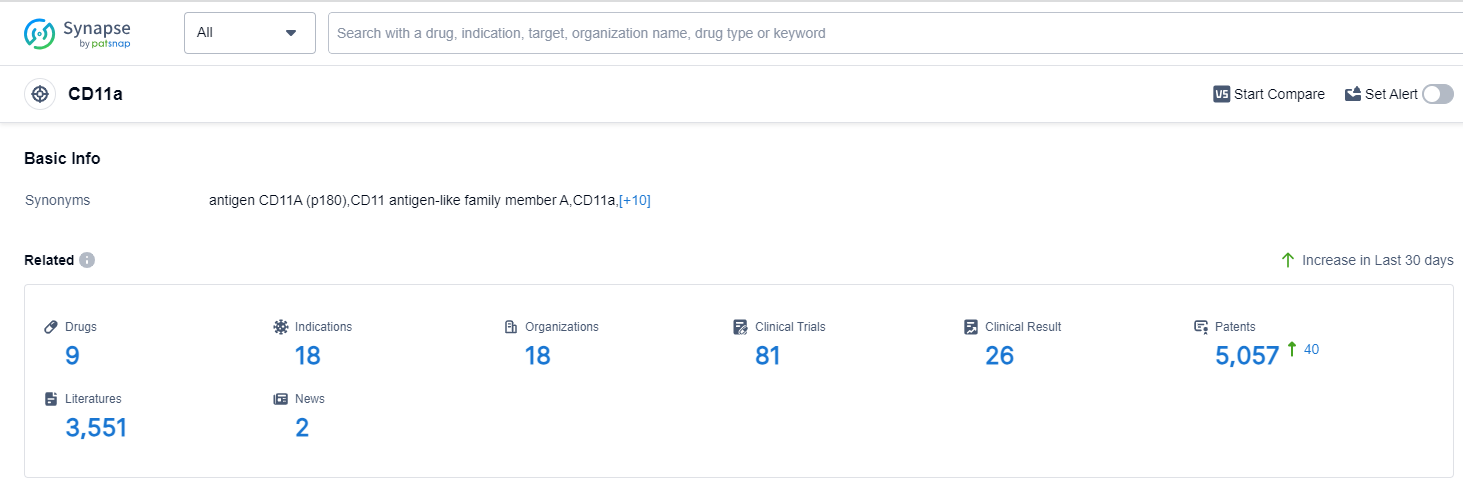
CD11a, also known as integrin alpha L, is a cell surface glycoprotein that plays a crucial role in the immune system. It is primarily expressed on leukocytes, including T cells, B cells, and monocytes. CD11a forms a heterodimer with CD18, known as LFA-1 (lymphocyte function-associated antigen-1), which mediates cell adhesion and migration. LFA-1 is involved in various immune responses, including leukocyte trafficking, antigen presentation, and T cell activation. Additionally, CD11a has been implicated in autoimmune diseases and inflammatory disorders, making it an attractive target for therapeutic interventions in the pharmaceutical industry.
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 9 CD11a drugs worldwide, from 18 organizations, covering 18 indications, and conducting 81 clinical trials.
The analysis of target CD11a reveals a competitive landscape with multiple companies focusing on its development. Novartis AG, Takeda Pharmaceutical Co., Ltd., and Merck KGaA are leading the way with approved drugs targeting CD11a. The indications for these drugs include psoriasis, dry eye syndromes, renal transplant rejection, graft vs host disease, and head and neck neoplasms. Monoclonal antibodies are the most prominent drug type in development for CD11a, indicating intense competition. The countries/locations with the highest development progress for CD11a include Australia, Switzerland, the United States, and the European Union. China has also shown progress with IND approval for a drug targeting CD11a. Overall, the current competitive landscape for target CD11a is dynamic, with potential for future development and advancements in the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In conclusion, efalizumab is a monoclonal antibody drug developed by Genentech, Inc. It targets CD11a and is primarily used for the treatment of psoriasis. Its approval in 2003 marked a significant advancement in the field of biomedicine, offering a targeted and effective treatment option for patients with psoriasis.