Decoding Etelcalcetide: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Etelcalcetide's R&D Progress
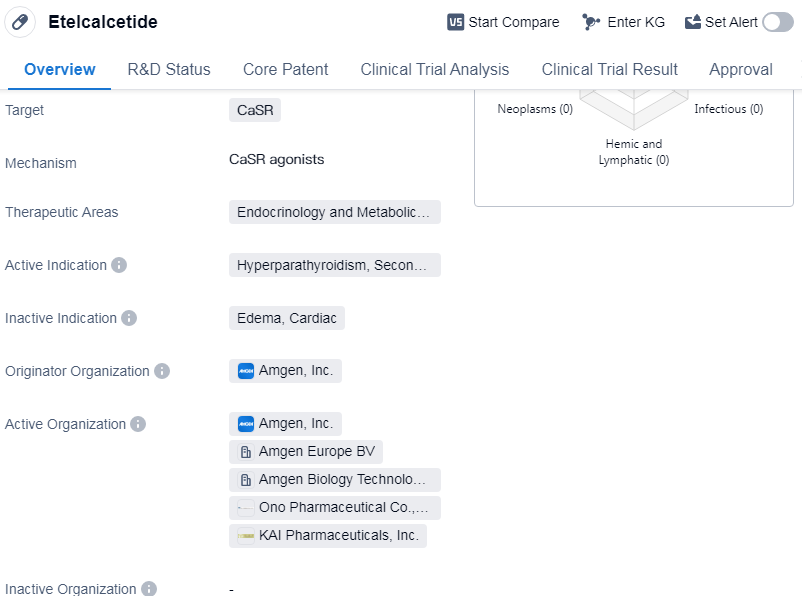
Etelcalcetide is a synthetic peptide drug that targets the calcium-sensing receptor (CaSR) and is primarily used in the treatment of hyperparathyroidism, specifically the secondary form. It falls under the therapeutic areas of endocrinology and metabolic diseases. The drug was developed by Amgen, Inc., a prominent organization in the pharmaceutical industry.
Etelcalcetide has successfully completed the highest phase of clinical trials and has received approval for use in multiple countries. Its first approval was granted in Iceland in October 2016. The drug has also been approved in China, indicating its global reach and potential for widespread use.
One notable aspect of Etelcalcetide is its classification as an orphan drug. Orphan drugs are medications developed to treat rare diseases that affect a small number of individuals. This classification often comes with certain regulatory benefits, such as extended market exclusivity and financial incentives, to encourage the development of drugs for rare diseases.
As a synthetic peptide, Etelcalcetide is a type of drug that is created through laboratory synthesis rather than being derived from natural sources. Peptides are short chains of amino acids that have various therapeutic effects. In the case of Etelcalcetide, its target, the calcium-sensing receptor, plays a crucial role in regulating calcium levels in the body.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Etelcalcetide: CaSR Agonists
CaSR agonists are a type of drug that acts on the CaSR in the body. The CaSR is a protein found on the surface of various cells, including those in the parathyroid glands, kidneys, and bones.
CaSR agonists are designed to bind to the CaSR and activate it, mimicking the effects of calcium. By activating the receptor, these agonists can modulate various physiological processes, such as the release of parathyroid hormone (PTH), which helps regulate calcium and phosphate levels in the blood.
In biomedicine, CaSR agonists are primarily used for the treatment of conditions related to abnormal calcium metabolism, such as primary hyperparathyroidism and secondary hyperparathyroidism in patients with chronic kidney disease. By activating the CaSR, these drugs help lower PTH levels and restore calcium balance.
CaSR agonists can also have potential applications in other areas of medicine, such as the treatment of osteoporosis and certain types of cancer. However, further research is needed to fully understand their therapeutic potential and ensure their safety and efficacy.
Overall, CaSR agonists are a class of drugs that target the calcium-sensing receptor to modulate calcium homeostasis and have potential therapeutic applications in various calcium-related disorders.
Drug Target R&D Trends for Etelcalcetide
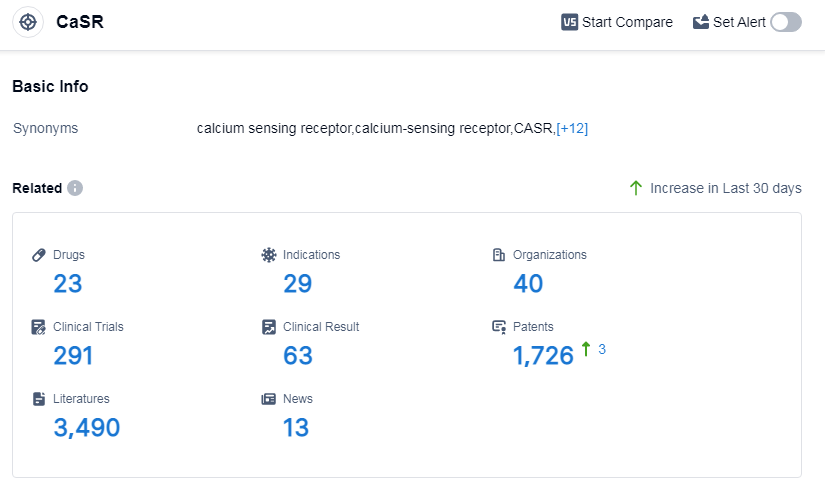
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 23 CaSR drugs worldwide, from 40 organizations, covering 29 indications, and conducting 291 clinical trials.
The analysis of the current competitive landscape of target CaSR reveals that Amgen, Inc., Kirin Holdings Co., Ltd., and Ascendis Pharma A/S are the companies growing fastest under this target. These companies have shown significant R&D progress in developing drugs targeting CaSR. Several drugs have been approved for indications such as secondary hyperparathyroidism, hypercalcemia, osteoporosis, and chronic kidney failure. The most rapidly progressing drug types are small molecule drugs and synthetic peptides, indicating intense competition in the market. Japan, European Union, China, Russia, and Iceland are the countries/locations developing fastest under the target CaSR, with Japan leading in terms of approved and inactive drugs. The future development of target CaSR holds promise for the pharmaceutical industry, with potential advancements in the treatment of various indications related to CaSR.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Etelcalcetide is a synthetic peptide drug developed by Amgen, Inc. It targets the calcium-sensing receptor and is primarily used in the treatment of secondary hyperparathyroidism. The drug has received approval in multiple countries, including Iceland and China, and is classified as an orphan drug. Its approval and therapeutic focus in the field of endocrinology and metabolic diseases highlight its potential impact in addressing rare conditions and improving patient outcomes.