Advances in Clinical Research on Neuraminidase Inhibitor
The surface of the influenza virus is distributed with three important proteins, namely, hemagglutinin (HA), neuraminidase (NA), and matrix protein (M2), each playing an important role in the transmission cycle of the influenza virus.
Firstly, the hemagglutinin (HA) of the influenza virus recognizes the sialic acid receptor on the host cell surface and binds to it, adsorbing on the cell membrane, and then the virus enters the cell for replication through receptor-mediated endocytosis. The matrix protein is responsible for forming ion channels in the viral envelope, facilitating the release and expression of viral genetic material. The replicated virus proteins and other components are transported to the cell surface, embedded in the cell membrane, and assembled into new virus particles. Finally, the mature influenza virus detaches from the host cell and continues to spread. At this stage, neuraminidase plays a very important role. The mature virus, before detaching from the host cell, maintains the final contact through the virus hemagglutinin and the cell sialic acid receptor. The function of neuraminidase is to hydrolyze the sialic acid receptor, cutting off the connection between the virus and the host cell, and forming a new infection. In addition, the surface of the newly formed virus particles is distributed with sialic acid receptors. The viruses recognize each other through hemagglutinin and aggregate, and the aggregated virus particles can be cleared by the human immune system. But neuraminidase can hydrolyze sialic acid residues to avoid the aggregation of new virus particles. Therefore, neuraminidase inhibitors (NIs) can block the life cycle of the virus and effectively control the further spread of the virus in the respiratory tract.
So far, two major classes of NA have been discovered, with a total of 10 subtypes (N1~N10). The first class includes N1, N4, N5, N8, and N10; the second class includes N2, N3, N6, N7, N9. The difference between the two lies in that the first class of NA has a "150 cavity" near the active center, while the latter does not. Although the homology between different subtypes of neuraminidase is not high, the 19 amino acids in their active center are highly conserved, and subsequent research is conducted on this basis.
Neuraminidase Competitive Landscape
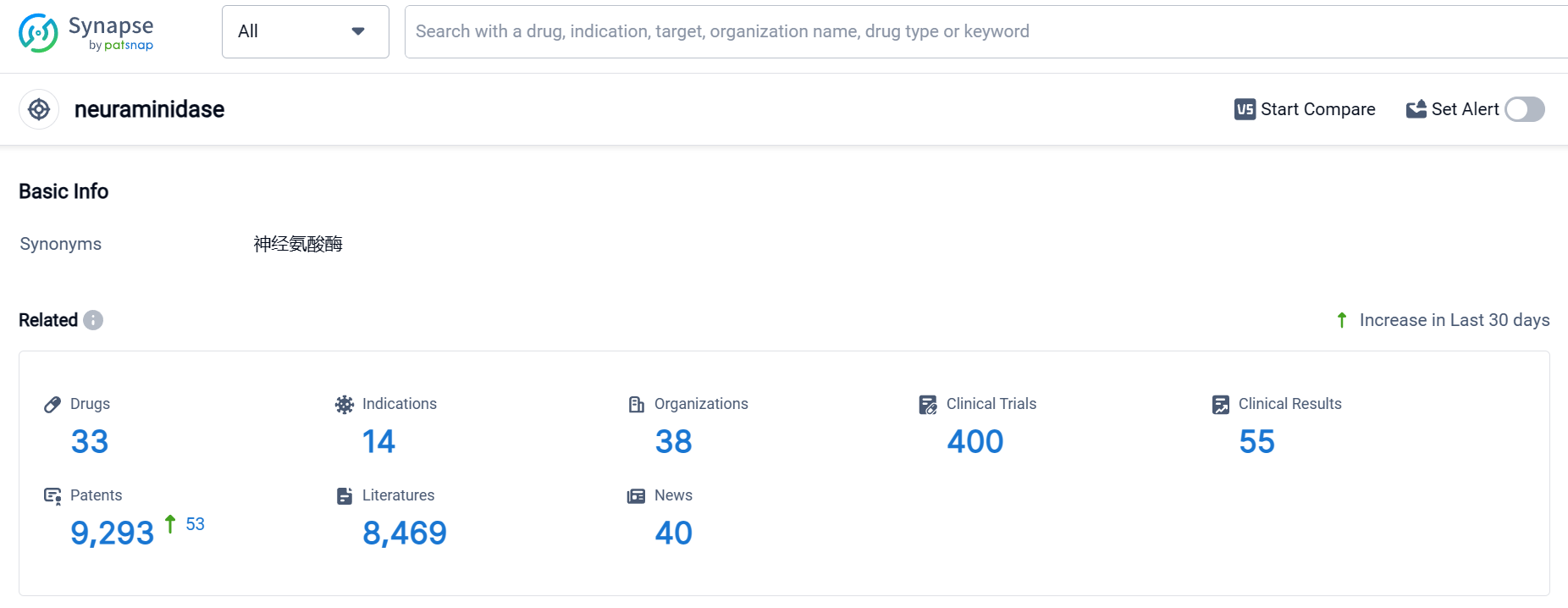
According to Patsnap Synapse, as of 5 Oct 2023, there are a total of 33 neuraminidase drugs worldwide, from 38 organizations, covering 14 indications, and conducting 400 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
Based on the analysis of the data provided, the current competitive landscape of the target neuraminidase in the pharmaceutical industry is characterized by the presence of multiple companies with drugs in various stages of development. BioCryst Pharmaceuticals, Inc., GSK Plc, Roche Holding AG, and Hunan Pharmaceutical Development Investment Group Co., Ltd. are among the companies with approved drugs for the target neuraminidase.
The indications for which drugs have been approved under the target neuraminidase include Influenza, Human, Influenza A virus infection, Influenza B virus infection, Fatigue, Cough, Nasal Obstruction, Headache, Arthralgia, and Musculoskeletal Pain.
The drug types progressing most rapidly under the current target neuraminidase are small molecule drugs and prophylactic vaccines. There are also some drugs with unknown drug types.
Countries/locations such as Japan, United States, China, European Union, Canada, Australia, Mexico, Norway, South Korea, Liechtenstein, Iceland, Russia, South Africa, New Zealand, Brazil, India, Chile, Israel, Lebanon, and Peru are developing drugs under the target neuraminidase.
Overall, the target neuraminidase presents a competitive landscape with multiple companies and a diverse range of drugs in various stages of development. The future development of this target will depend on the progress of these companies and the approval of drugs for relevant indications.
The world's first marketed Neuraminidase inhibitor:Zanamivir
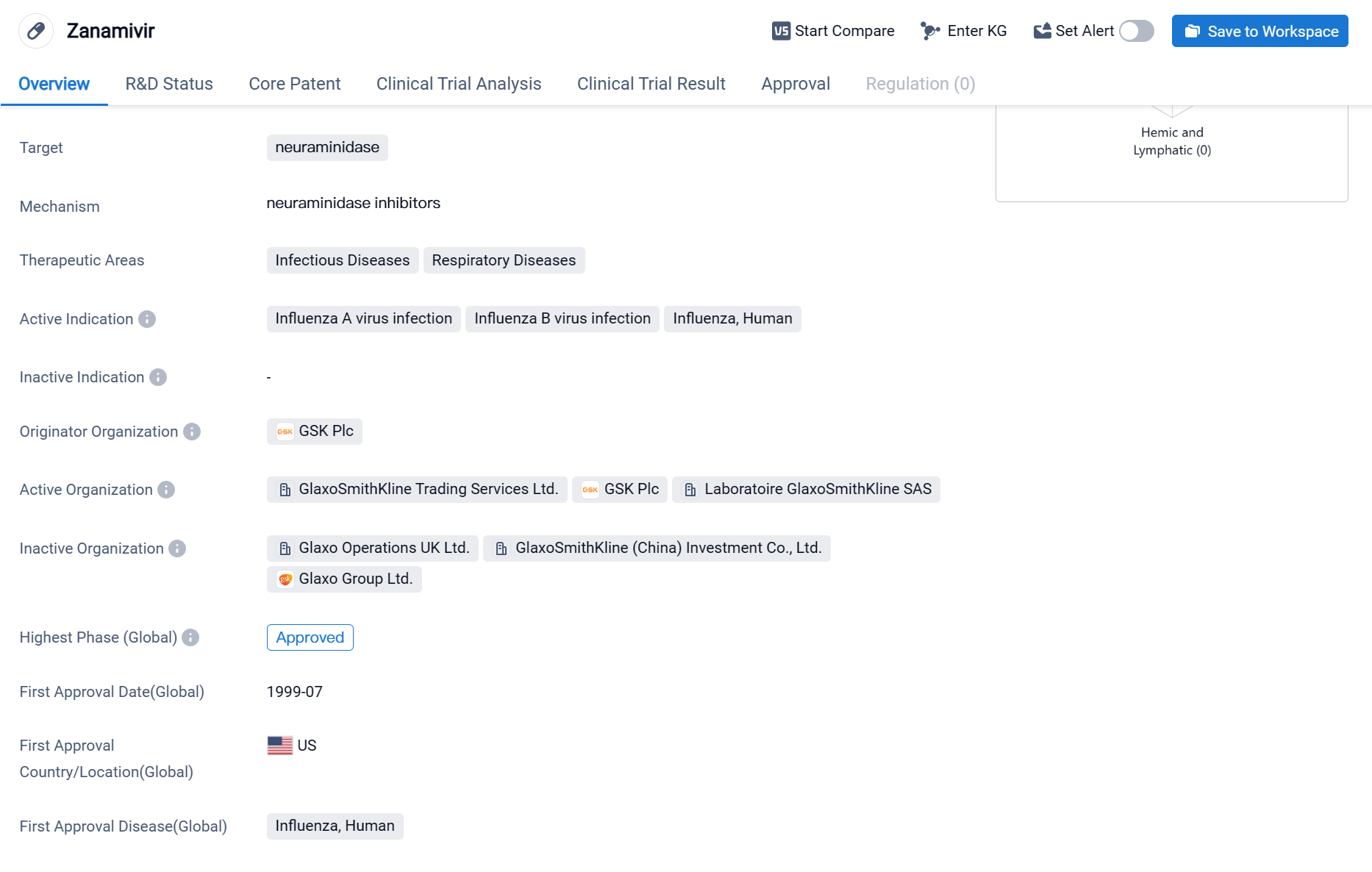
Zanamivir is a small molecule drug that falls under the therapeutic areas of Infectious Diseases and Respiratory Diseases. It specifically targets neuraminidase, an enzyme found on the surface of the influenza virus. The drug is primarily indicated for the treatment of Influenza A virus infection, Influenza B virus infection, and Influenza in humans.
Zanamivir was first approved in the United States in July 1999, making it one of the early drugs in the market for the treatment of influenza. The drug was developed by GSK Plc, a renowned pharmaceutical company. It is important to note that Zanamivir has reached the highest phase of approval globally.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
As a neuraminidase inhibitor, Zanamivir works by blocking the activity of the neuraminidase enzyme, which is crucial for the release of newly formed viral particles from infected cells. By inhibiting this enzyme, the drug helps to reduce the spread of the influenza virus within the body, thereby alleviating symptoms and shortening the duration of the illness.
Influenza is a highly contagious respiratory illness that affects millions of people worldwide each year. It can lead to severe complications, especially in vulnerable populations such as the elderly, young children, and individuals with weakened immune systems. Zanamivir provides a valuable treatment option for healthcare professionals to manage and control influenza infections.
The approval of Zanamivir in both the United States and China highlights its global significance and recognition by regulatory authorities. Its long-standing presence in the market since 1999 demonstrates its established track record and widespread use in the treatment of influenza.
In conclusion, Zanamivir is a small molecule drug developed by GSK Plc that targets neuraminidase, an enzyme involved in the release of influenza virus particles. It is approved for the treatment of various types of influenza infections in both the United States and China. With its proven efficacy and safety, Zanamivir plays a crucial role in managing and mitigating the impact of influenza on public health.