Deep Scientific Insights on Eteplirsen's R&D Progress, Mechanism of Action, and Drug Target
Eteplirsen's R&D Progress
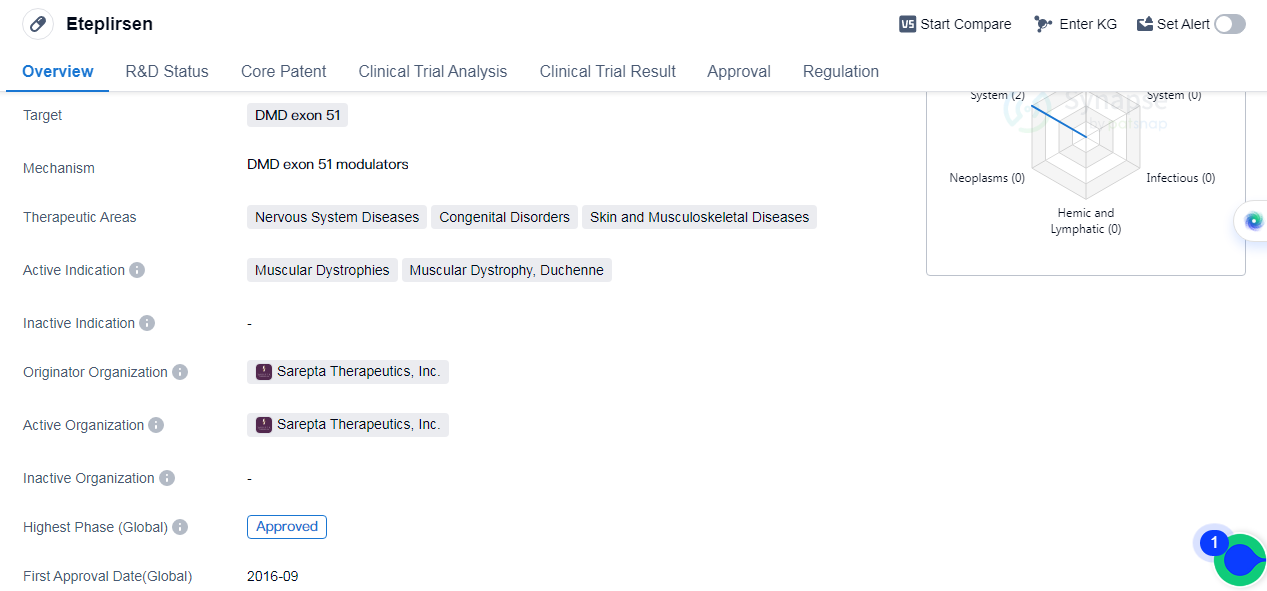
Eteplirsen is an antisense oligonucleotide drug developed by Sarepta Therapeutics, Inc. It falls under the therapeutic area of nervous system diseases, congenital disorders, and skin and musculoskeletal diseases. The drug specifically targets Duchenne muscular dystrophy (DMD) exon 51 and is indicated for the treatment of muscular dystrophies, specifically Duchennemuscular dystrophy.
Eteplirsen received its first approval in the United States in September 2016, making it available for patients in that country. The drug has also reached the highest phase of development globally, which is the approved stage. In China, however, it is currently in phase 3 of clinical trials, indicating that it is still undergoing testing and evaluation in that market.
Regulatory-wise, Eteplirsen has been granted several designations to expedite its development and approval process. These include accelerated approval, fast-track, and orphan drug status. Accelerated approval allows for the conditional approval of drugs that address serious conditions and provide meaningful benefits over existing treatments. Fast-track designation is given to drugs that have the potential to address unmet medical needs and expedite their development and review. Lastly, orphan drug status is granted to drugs that target rare diseases, providing incentives for their development.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Eteplirsen: DMD Exon 51 Modulators
DMD exon 51 modulators are a type of drug that is used in the treatment of DMD. DMD is a genetic disorder characterized by the absence of a protein called dystrophin, which is essential for muscle function. Exon 51 is a specific region of the dystrophin gene that is often mutated in individuals with DMD.
DMD exon 51 modulators work by targeting and modifying the splicing of exon 51 during the production of dystrophin. This modulation allows for the production of a shorter but partially functional dystrophin protein, which can help improve muscle function in individuals with DMD.
These modulators are designed to be specific to exon 51, as mutations in this region are relatively common in DMD patients. By targeting exon 51, these drugs aim to restore some level of dystrophin production and potentially slow down the progression of muscle degeneration in individuals with DMD.
It is important to note that DMD exon 51 modulators are not a cure for DMD, but they can provide some therapeutic benefit by addressing the underlying genetic cause of the disease. These drugs are typically used in combination with other supportive therapies to manage the symptoms and improve the quality of life for individuals with DMD.
Drug Target R&D Trends for Eteplirsen
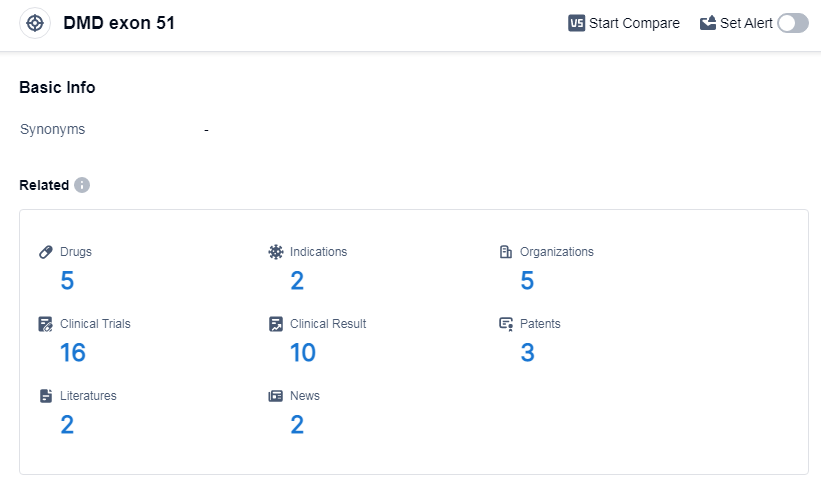
According to Patsnap Synapse, as of 7 Sep 2023, there are a total of 5 DMD exon 51 drugs worldwide, from 5 organizations, covering 2 indications, and conducting 16 clinical trials.
The analysis of the current competitive landscape and future development of target DMD exon 51 reveals that Sarepta Therapeutics, Inc. is the leading company in terms of R&D progress. Drugs targeting DMD exon 51 have been approved for the indication of muscular dystrophy, Duchenne, indicating successful development in this area. Antisense oligonucleotides and gene therapy are the most rapidly progressing drug types, with potential competition from biosimilars. The countries or locations developing fastest include the United States, China, and various European countries, with China showing notable progress. Overall, the analysis suggests a promising future for the development of drugs targeting DMD exon 51, with a focus on addressing the unmet medical needs in muscular dystrophy, and Duchenne and exploring innovative treatment approaches.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Eteplirsen is an antisense oligonucleotide drug developed by Sarepta Therapeutics, Inc. It targets DMD exon 51 and is indicated for the treatment of muscular dystrophies, specifically Duchenne muscular dystrophy. The drug has received approval in the United States and is currently in phase 3 of clinical trials in China. It has been granted several regulatory designations, including accelerated approval, fast-track, and orphan drug status.