Decoding Lanadelumab: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Lanadelumab's R&D Progress
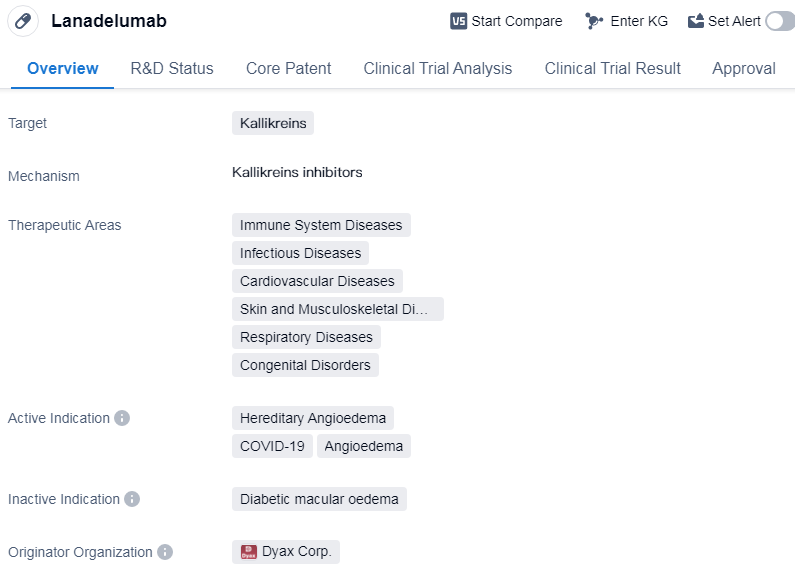
Lanadelumab is a monoclonal antibody drug that targets kallikreins. It is primarily used in the treatment of hereditary angioedema, a rare genetic disorder that causes recurrent episodes of swelling in various parts of the body. However, it has also shown potential in the treatment of other conditions such as COVID-19 and angioedema.
The drug was developed by Dyax Corp., a pharmaceutical company specializing in biomedicine. It received its first approval in the United States in August 2018, making it available for use in the country. Lanadelumab has also obtained approval in China, indicating its global reach and potential for widespread use.
In terms of therapeutic areas, Lanadelumab is primarily focused on immune system diseases. However, it has also shown promise in the treatment of infectious diseases, cardiovascular diseases, skin and musculoskeletal diseases, respiratory diseases, and congenital disorders. This wide range of therapeutic areas suggests the drug's versatility and potential for addressing various medical conditions.
Lanadelumab has undergone rigorous testing and evaluation, reaching the highest phase of approval globally. This indicates that it has successfully completed clinical trials and demonstrated its safety and efficacy. The drug has also received regulatory designations such as priority review, fast track, orphan drug, and breakthrough therapy. These designations highlight the drug's potential to address unmet medical needs and expedite its development and approval process.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Lanadelumab: Kallikreins inhibitors
Kallikreins inhibitors are a class of compounds or drugs that specifically target and inhibit the activity of kallikreins. Kallikreins are a group of enzymes involved in various physiological processes, including blood pressure regulation, inflammation, and tissue remodeling. They belong to the serine protease family and play a role in the activation of certain proteins and peptides.
From a biomedical perspective, kallikreins inhibitors are of interest due to their potential therapeutic applications. In certain diseases or conditions, the activity of kallikreins can be dysregulated, leading to imbalances and pathological processes. By inhibiting the activity of kallikreins, these inhibitors can help restore the normal physiological balance and potentially alleviate the associated symptoms or progression of the disease.
Kallikreins inhibitors have been studied in the context of various medical conditions, including cancer, cardiovascular diseases, and inflammatory disorders. For example, in cancer, kallikreins inhibitors may be explored as a potential treatment strategy to inhibit tumor growth and metastasis, as kallikreins have been implicated in these processes. In cardiovascular diseases, kallikreins inhibitors may be investigated for their potential to regulate blood pressure and prevent excessive tissue remodeling. Inflammatory disorders, such as asthma or rheumatoid arthritis, may also benefit from kallikreins inhibitors by reducing inflammation and tissue damage.
Overall, kallikreins inhibitors are compounds or drugs that selectively inhibit the activity of kallikreins, offering potential therapeutic benefits in various disease contexts.
Drug Target R&D Trends for Lanadelumab
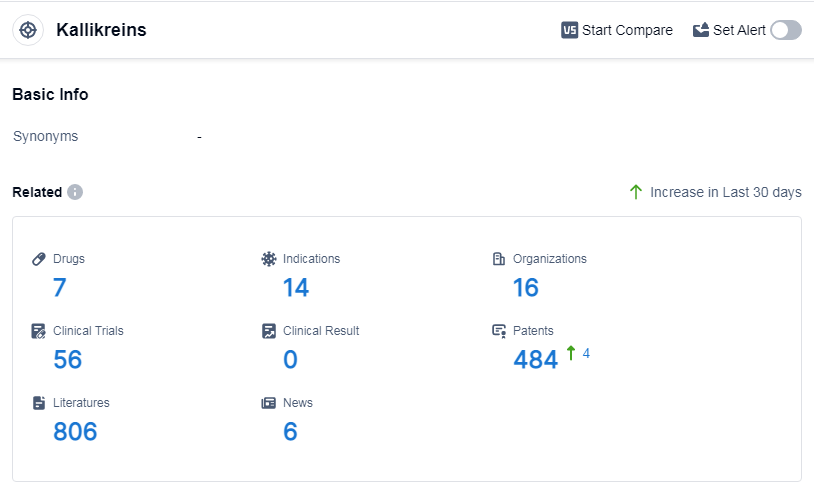
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 7 Kallikreins drugs worldwide, from 16 organizations, covering 14 indications, and conducting 56 clinical trials.
The analysis of the current competitive landscape of target Kallikreins reveals that Takeda Pharmaceutical Co., Ltd. is the company with the highest stage of development, with drugs in various phases. Hereditary Angioedema is the indication with the highest number of approved drugs, indicating a focus on developing drugs for this indication. Monoclonal antibodies, Recombinant proteins, and Small molecule drugs are progressing rapidly, indicating intense competition in these categories. The United States, European Union, and China are the countries/locations with significant progress in R&D for drugs targeting Kallikreins. Overall, the target Kallikreins shows a promising future in terms of R&D and development of drugs for various indications.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
Overall, Lanadelumab is a monoclonal antibody drug developed by Dyax Corp. that targets kallikreins. It has been approved for the treatment of hereditary angioedema and has shown potential in addressing other medical conditions such as COVID-19 and angioedema. With its wide range of therapeutic areas and regulatory designations, Lanadelumab has the potential to make a significant impact in the field of biomedicine.