Decoding Pyridostigmine bromide: A Comprehensive Study of its R&D Trends
Pyridostigmine bromide's R&D Progress
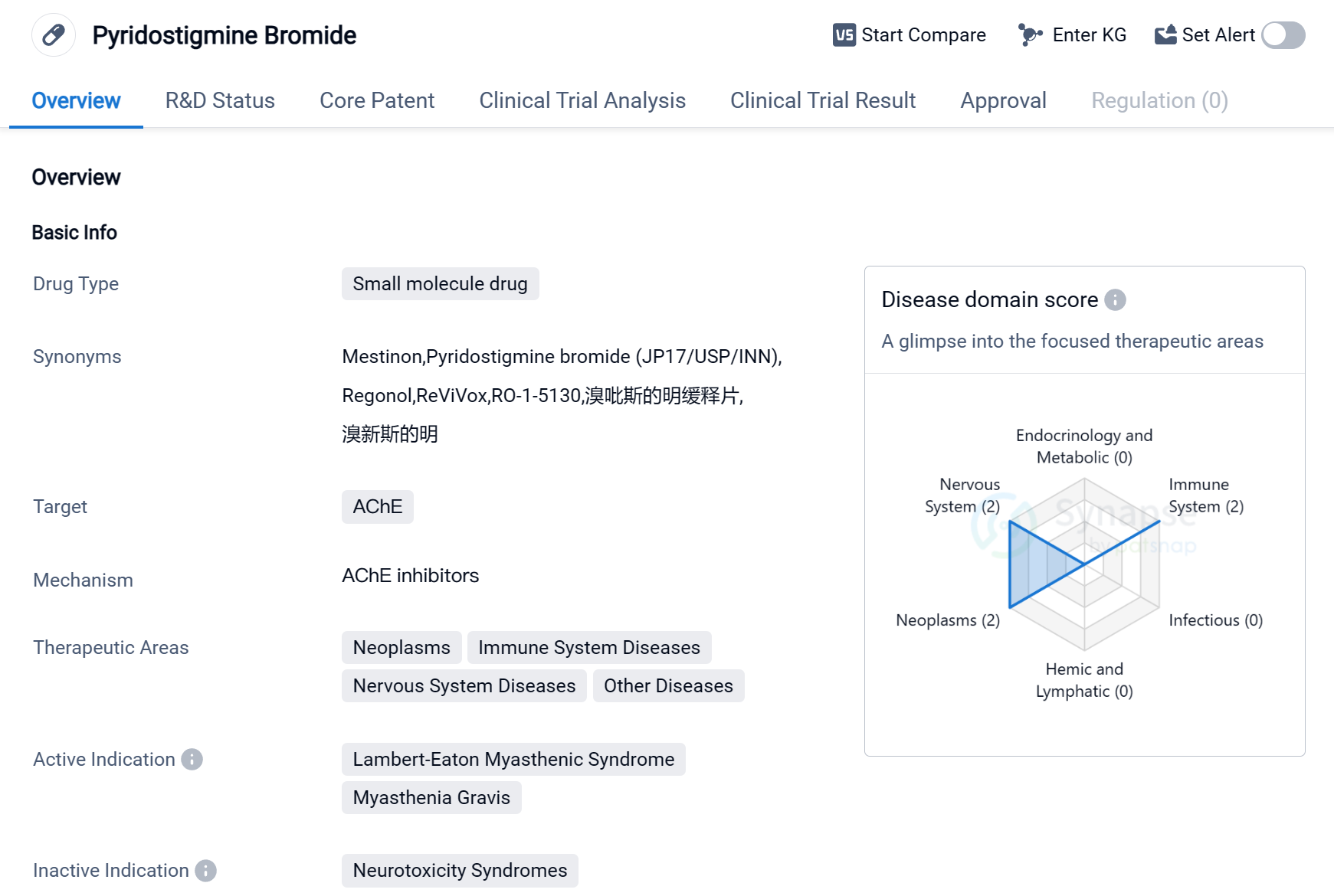
Pyridostigmine Bromide is a small molecule drug that primarily targets the enzyme acetylcholinesterase (AChE). It is used in the treatment of various diseases related to the immune system, nervous system, neoplasms, and other conditions. The drug has been approved for the treatment of Lambert-Eaton Myasthenic Syndrome (LEMS) and Myasthenia Gravis.
LEMS is a rare autoimmune disorder that affects the neuromuscular junction, leading to muscle weakness and fatigue. Myasthenia Gravis is also an autoimmune disease that causes muscle weakness and fatigue, primarily affecting the voluntary muscles. Pyridostigmine Bromide helps in managing the symptoms of these conditions by inhibiting the breakdown of acetylcholine, a neurotransmitter involved in muscle contraction.
The drug was first approved in the United States in April 1955, making it one of the older drugs in the market. It is developed and marketed by Bausch Health Americas, Inc., a pharmaceutical company specializing in various therapeutic areas. Pyridostigmine Bromide has also received approval in China, indicating its global recognition and acceptance.
As a small molecule drug, Pyridostigmine Bromide is likely to have a well-established formulation and manufacturing process. It may be available in various dosage forms, such as tablets or oral solutions, to cater to different patient needs.
Given its therapeutic areas, Pyridostigmine Bromide may have potential applications in the treatment of other diseases beyond LEMS and Myasthenia Gravis. However, further research and clinical trials would be required to explore its efficacy and safety in these areas.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pyridostigmine bromide: AChE inhibitor
AChE inhibitors, also known as acetylcholinesterase inhibitors, are a class of drugs that inhibit the activity of the enzyme acetylcholinesterase. Acetylcholinesterase is responsible for breaking down the neurotransmitter acetylcholine in the synaptic cleft, which is necessary for proper nerve signal transmission. By inhibiting the activity of this enzyme, AChE inhibitors increase the levels of acetylcholine in the brain, leading to enhanced cholinergic neurotransmission.
From a biomedical perspective, AChE inhibitors are commonly used in the treatment of various conditions, particularly those affecting the central nervous system. They are primarily used in the management of Alzheimer's disease, a progressive neurodegenerative disorder characterized by a decline in cognitive function. By increasing acetylcholine levels, AChE inhibitors can help improve memory, thinking, and overall cognitive abilities in individuals with Alzheimer's disease.
Additionally, AChE inhibitors may also be used in the treatment of other conditions such as myasthenia gravis, a neuromuscular disorder, and certain types of glaucoma. In myasthenia gravis, AChE inhibitors help improve muscle strength by enhancing neuromuscular transmission. In glaucoma, these inhibitors can reduce intraocular pressure by increasing the outflow of aqueous humor.
It is important to note that the use of AChE inhibitors may be associated with side effects such as nausea, vomiting, diarrhea, and muscle cramps. These medications should be used under the supervision of a healthcare professional and dosages should be carefully adjusted to minimize adverse effects.
Drug Target R&D Trends for Pyridostigmine bromide
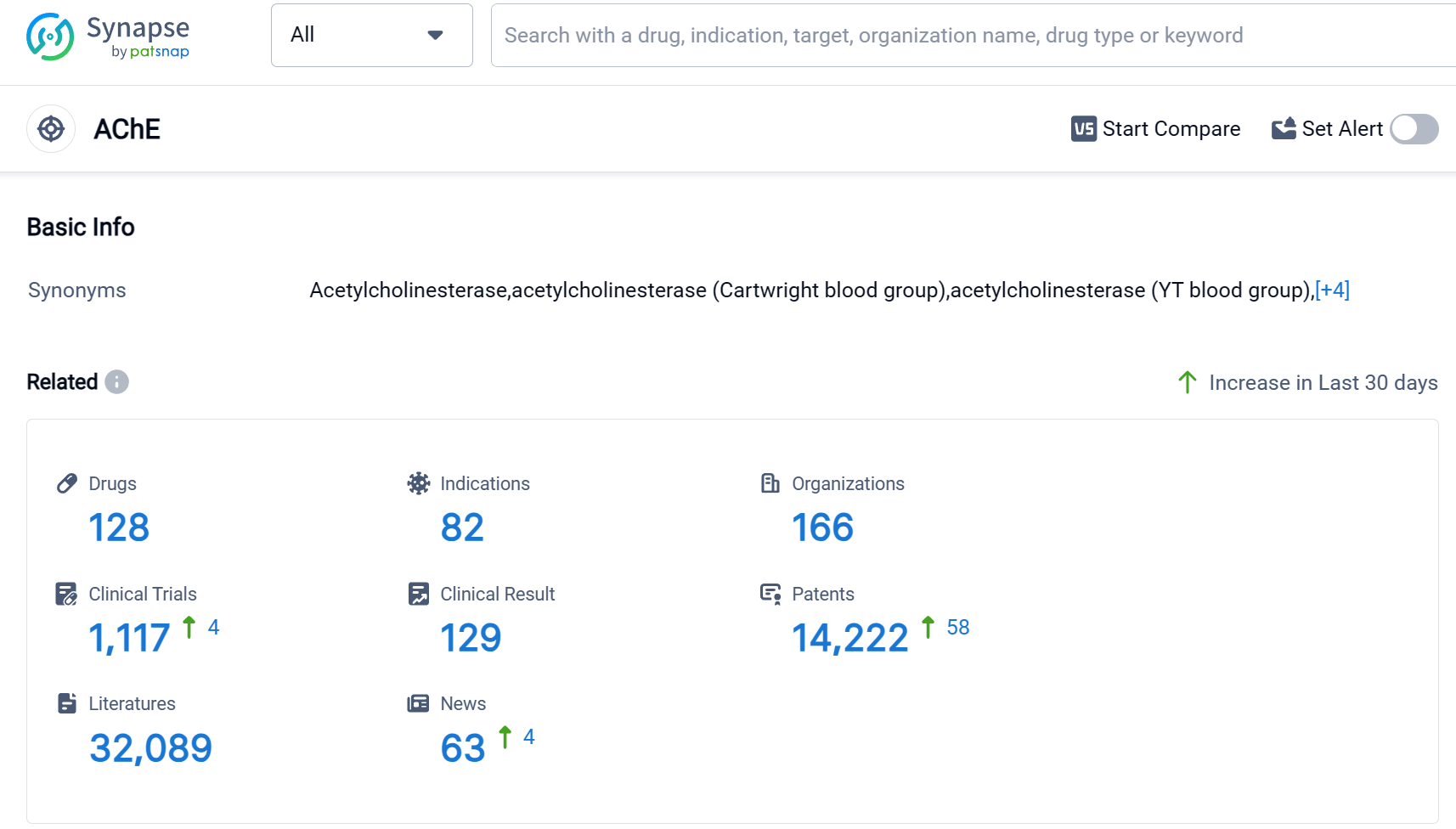
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 128 AChE drugs worldwide, from 166 organizations, covering 82 indications, and conducting 1117 clinical trials.
The analysis of the target AChE reveals a competitive landscape with multiple companies actively involved in R&D. AbbVie, Inc., Shanghai Pharmaceuticals Holding Co., Ltd., Pfizer Inc., Zeria Pharmaceutical Co., Ltd., and Eisai Co., Ltd. are among the companies growing fastest in this area. The approved drugs for the target AChE cover indications such as Myasthenia Gravis, Alzheimer Disease, Poisoning, Glaucoma, and more. Small molecule drugs dominate the drug types progressing rapidly under the target AChE, indicating intense competition. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target AChE, with China showing significant progress. Overall, the target AChE presents a promising area for pharmaceutical development with a diverse range of indications and drug types.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pyridostigmine Bromide is a small molecule drug developed by Bausch Health Americas, Inc. It primarily targets AChE and is approved for the treatment of Lambert-Eaton Myasthenic Syndrome and Myasthenia Gravis. With its long history of approval and global recognition, the drug holds promise in managing the symptoms of these conditions. Further research may uncover its potential in treating other diseases related to the immune and nervous systems, as well as neoplasms and other conditions.