Deep Scientific Insights on Pramipexole dihydrochloride's R&D Progress
Pramipexole dihydrochloride's R&D Progress
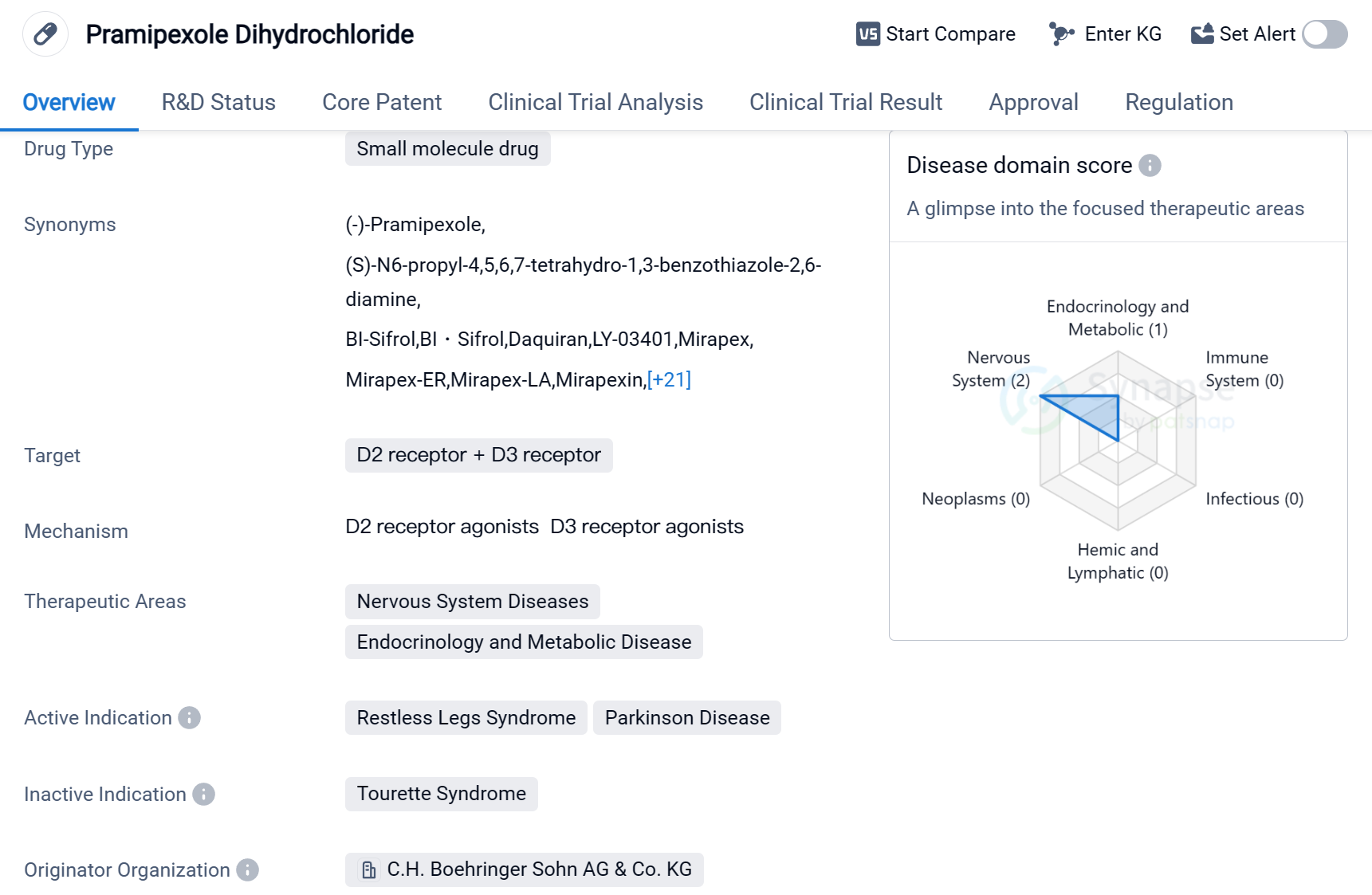
Pramipexole Dihydrochloride is a small molecule drug that targets the D2 receptor and D3 receptor in the field of biomedicine. It is primarily used to treat nervous system diseases and endocrinology and metabolic diseases. The drug has been approved for the treatment of Restless Legs Syndrome and Parkinson's Disease.
The originator organization of Pramipexole Dihydrochloride is C.H. Boehringer Sohn AG & Co. KG. It received its first approval in the United States in July 1997, making it available to patients in that country. The drug has also received approval in China.
Pramipexole Dihydrochloride has reached the highest phase of development in global markets. This indicates that it has successfully completed clinical trials and met the necessary regulatory requirements for market authorization.
In terms of regulation, Pramipexole Dihydrochloride has undergone priority review, which suggests that it addresses an unmet medical need or provides a significant improvement over existing treatments. Additionally, it has been designated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Pramipexole dihydrochloride: D2 receptor agonists and D3 receptor agonists
D2 receptor agonists and D3 receptor agonists are types of drugs that target specific receptors in the brain called D2 receptors and D3 receptors, respectively.
From a biomedical perspective, D2 receptors and D3 receptors are subtypes of dopamine receptors, which are a type of G protein-coupled receptor found in the central nervous system. These receptors play a crucial role in regulating dopamine neurotransmission, which is involved in various physiological and psychological processes.
D2 receptor agonists are drugs that bind to and activate D2 receptors, leading to an increase in dopamine signaling. By stimulating D2 receptors, these agonists can have therapeutic effects in conditions such as Parkinson's disease, where there is a deficiency of dopamine. They can help alleviate motor symptoms and improve overall motor function.
Similarly, D3 receptor agonists are drugs that specifically target and activate D3 receptors. The activation of D3 receptors can modulate dopamine release and transmission in specific brain regions, potentially influencing various neurological and psychiatric disorders. Research suggests that D3 receptor agonists may have potential therapeutic applications in conditions such as schizophrenia, addiction, and depression.
It's important to note that the specific effects and clinical applications of D2 receptor agonists and D3 receptor agonists may vary depending on the drug compound and the targeted disorder. These drugs are typically developed and studied in preclinical and clinical trials to evaluate their safety, efficacy, and potential side effects before being approved for medical use.
Drug Target R&D Trends for Pramipexole dihydrochloride
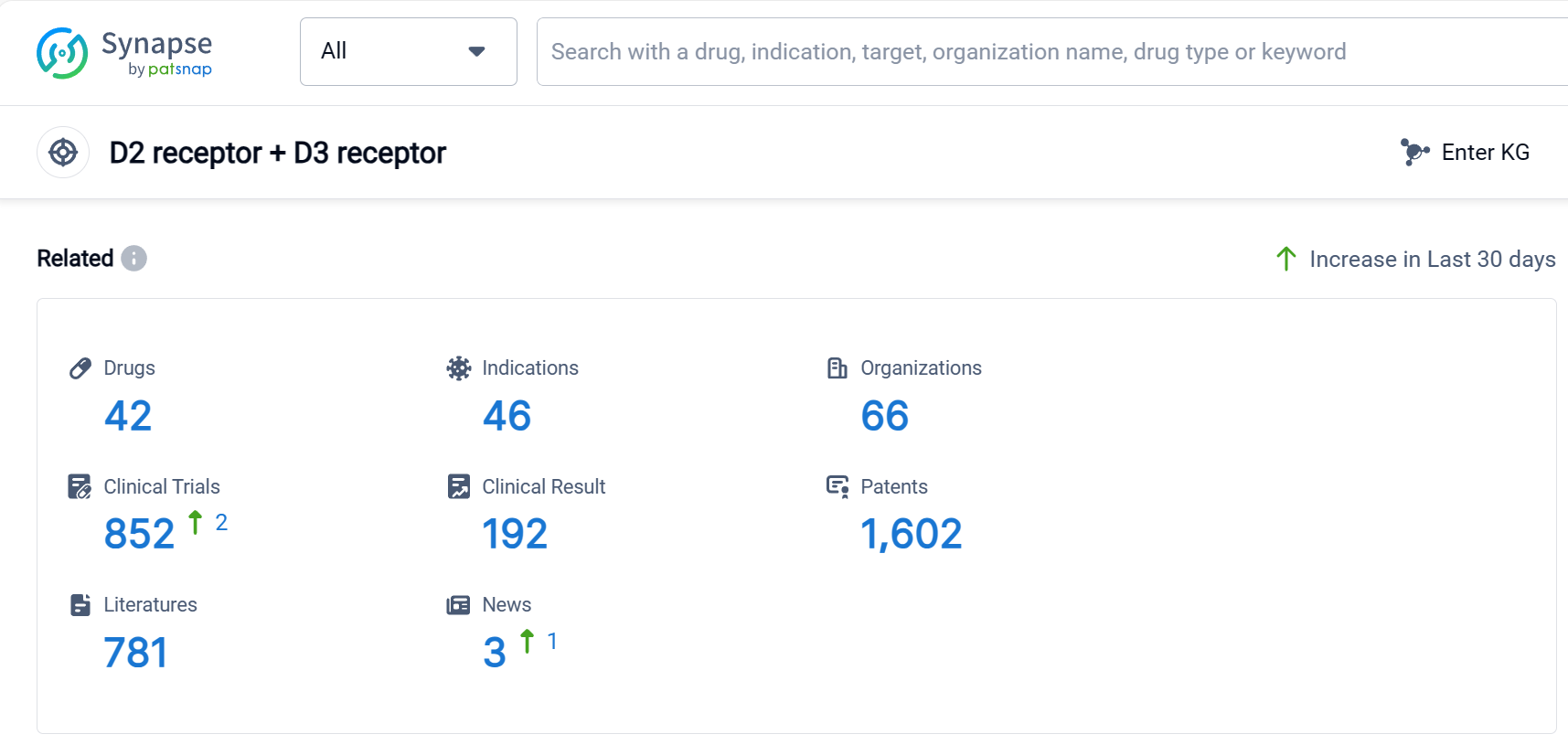
According to Patsnap Synapse, as of 9 Sep 2023, there are a total of 42 D2 receptor + D3 receptor drugs worldwide, from 66 organizations, covering 46 indications, and conducting 852 clinical trials.
In conclusion, the target D2 receptor + D3 receptor is being actively pursued by several pharmaceutical companies, including Sanofi, Otsuka Holdings Co., Ltd., and Reviva Pharmaceuticals Holdings, Inc. Drugs targeting this receptor have been approved for indications such as schizophrenia, Parkinson's disease, depressive disorder major, bipolar disorder, and Tourette syndrome, among others. Small molecule drugs are progressing most rapidly under this target, with a significant number of approved and preclinical drugs. The United States, European Union, China, and Japan are the countries/locations with the highest development activity, with China showing notable progress. Overall, the competitive landscape for the target D2 receptor + D3 receptor is dynamic, with potential for further development and innovation in the future.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Pramipexole Dihydrochloride is a small molecule drug that targets the D2 receptor and D3 receptor. It has been approved for the treatment of Restless Legs Syndrome and Parkinson's Disease. The drug was first approved in the United States in 1997 and has also received approval in China. Pramipexole Dihydrochloride has reached the highest phase of development globally, indicating its successful completion of clinical trials. It has undergone priority review and has been designated as an orphan drug, highlighting its potential to address unmet medical needs and rare diseases.