Penicillamine: Detailed Review of its Transformative R&D Success
Penicillamine's R&D Progress
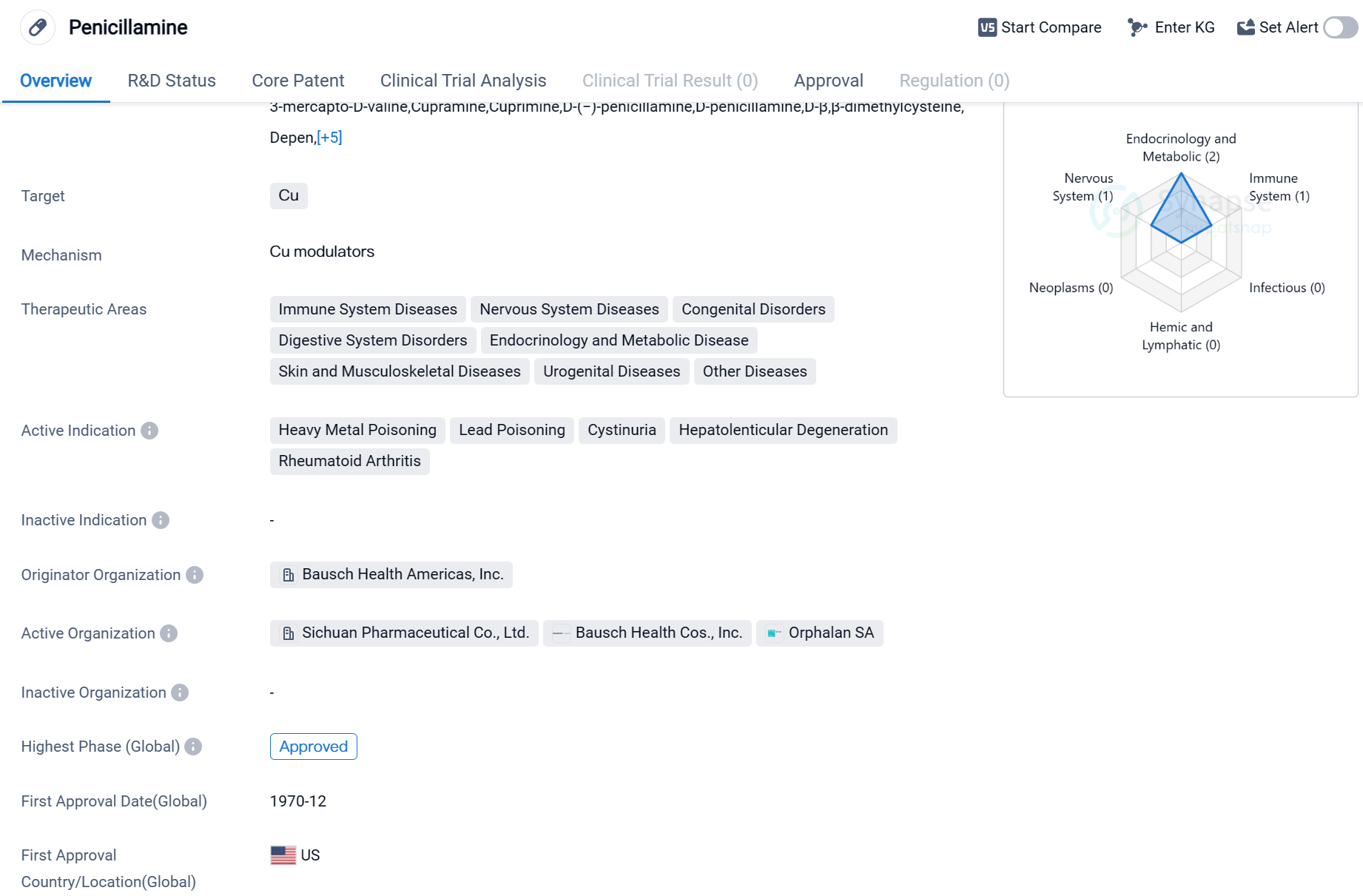
Penicillamine is a small molecule drug that primarily targets copper (Cu) in the body. It is used to treat a wide range of therapeutic areas including immune system diseases, nervous system diseases, congenital disorders, digestive system disorders, endocrinology and metabolic diseases, skin and musculoskeletal diseases, urogenital diseases, and other diseases.
The active indications for Penicillamine include heavy metal poisoning, lead poisoning, cystinuria, hepatolenticular degeneration, and rheumatoid arthritis. These conditions are characterized by an imbalance or excess of copper or other heavy metals in the body, leading to various health issues.
The drug was first approved in the United States in December 1970, making it a well-established medication with a long history of use. It is also approved in China, indicating its global recognition and acceptance.
Penicillamine was developed by Bausch Health Americas, Inc., a prominent organization in the pharmaceutical industry. As the originator organization, Bausch Health Americas, Inc. holds the rights to the drug and is responsible for its production, distribution, and marketing.
The highest R&D phase of this drug is approved globally. This indicates that it has met the necessary regulatory requirements and has been deemed suitable for use in treating the specified conditions.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Penicillamine: Cu modulators
Cu modulators refer to compounds or substances that can modulate or regulate the activity of copper (Cu) ions in biological systems. Copper is an essential trace element that plays crucial roles in various physiological processes, including enzymatic reactions, cellular metabolism, and the functioning of the immune system. Cu modulators can either enhance or inhibit the activity of copper ions depending on their specific mechanism of action.
From a biomedical perspective, Cu modulators can have therapeutic potential in the treatment of diseases associated with copper dysregulation, such as Wilson's disease, Menkes disease, and certain types of cancer. For example, in Wilson's disease, which is characterized by impaired copper transport and accumulation of copper in various tissues, Cu modulators can help restore copper homeostasis by facilitating copper excretion or reducing copper uptake.
Furthermore, Cu modulators can also be utilized in diagnostic applications. They can be employed as imaging agents to detect and visualize copper accumulation in tissues or cells, aiding in the diagnosis and monitoring of copper-related disorders.
Overall, Cu modulators play a significant role in regulating copper levels and can have therapeutic and diagnostic implications in various biomedical contexts.
Drug Target R&D Trends for Penicillamine
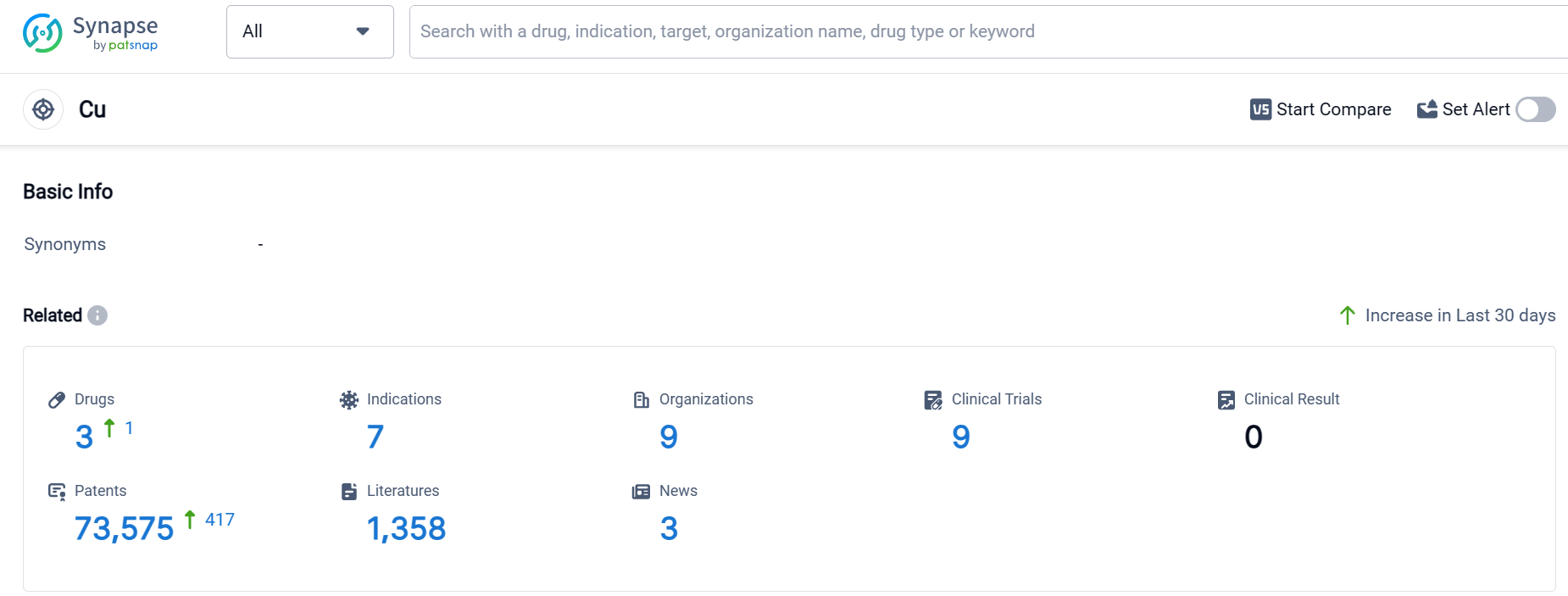
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 3 Cu drugs worldwide, from 9 organizations, covering 7 indications, and conducting 9 clinical trials.
Based on the analysis of the provided data, the current competitive landscape for target Cu shows a diverse range of companies and countries involved in its development. Bausch Health Cos., Inc. and Sichuan Pharmaceutical Co., Ltd. are leading in terms of having drugs in the Approved phase. The indications targeted by these drugs include Hepatolenticular Degeneration, Heavy Metal Poisoning, Cystinuria, Rheumatoid Arthritis, and Lead Poisoning. The drug types progressing rapidly are Small molecule drugs and Chemical drugs. The countries/locations with significant progress include the United States, China, France, European Union, and several others. Further information regarding the R&D progress of the companies and the research and development institutions involved in biosimilars is required to provide a more comprehensive analysis of the current competitive landscape and future development of target Cu.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Penicillamine is a small molecule drug that targets copper and is used to treat a wide range of diseases and disorders. It has been approved for use in multiple countries, including the United States and China, and was developed by Bausch Health Americas, Inc. Its active indications include heavy metal poisoning, lead poisoning, cystinuria, hepatolenticular degeneration, and rheumatoid arthritis. The drug has a long history of use, with its first approval dating back to 1970.