Decoding tenofovir alafenamide fumarate: A Comprehensive Study of its R&D Trends and Mechanism on Drug Target
Tenofovir alafenamide fumarate's R&D Progress
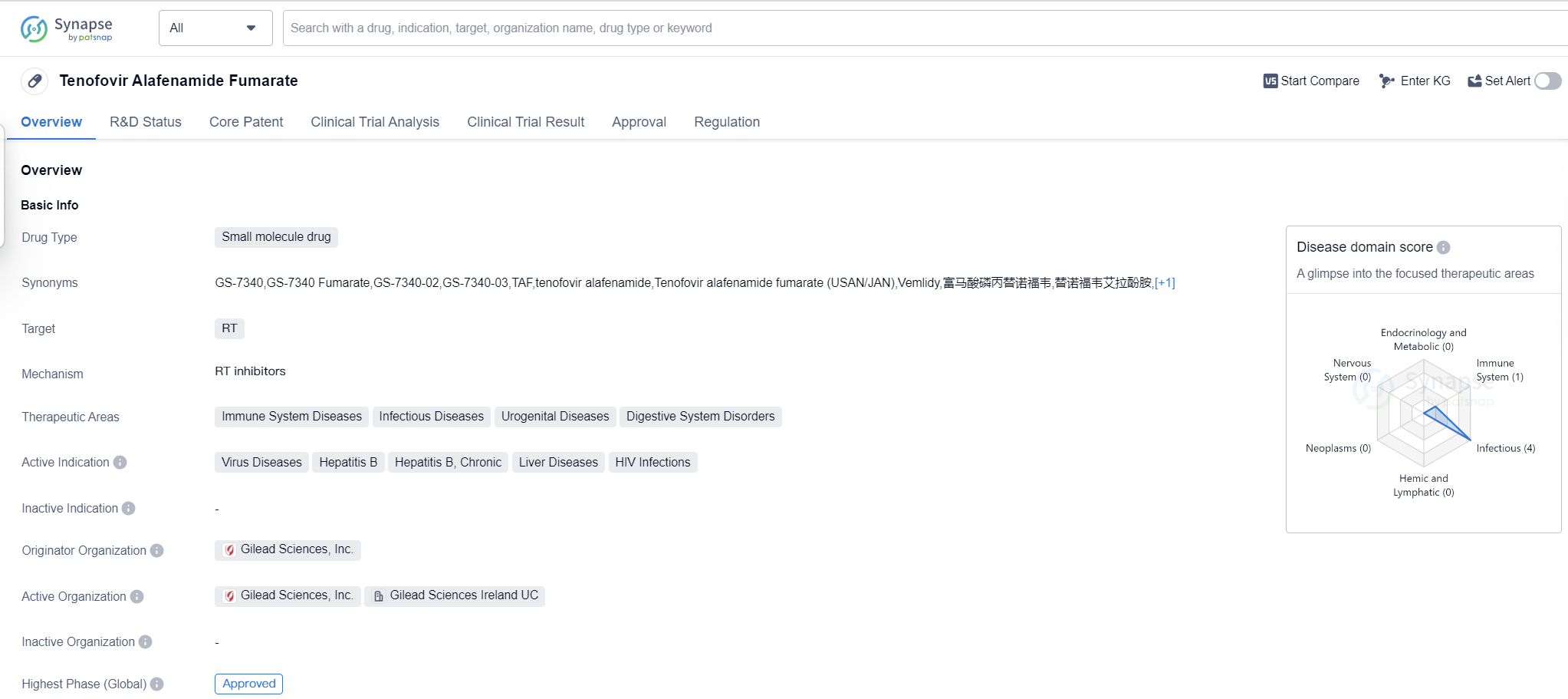
Tenofovir Alafenamide Fumarate is a small molecule drug that targets the reverse transcriptase (RT) enzyme. It is primarily used in the treatment of various immune system diseases, infectious diseases, urogenital diseases, and digestive system disorders. The drug has shown efficacy in treating virus diseases, chronic hepatitis B, liver diseases, and HIV infections.
The originator organization of Tenofovir Alafenamide Fumarate is Gilead Sciences, Inc., a renowned pharmaceutical company. The drug has received approval for use in multiple countries, including the United States, where it was first approved in November 2016.
Tenofovir Alafenamide Fumarate has reached the highest phase of development which is approved globally. The drug's approval in multiple countries indicates its potential to meet global medical needs.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for tenofovir alafenamide fumarate: RT inhibitors
RT inhibitors, also known as reverse transcriptase inhibitors, are a class of drugs used in the treatment of viral infections, particularly those caused by retroviruses such as HIV. Reverse transcriptase is an enzyme that retroviruses use to convert their RNA genome into DNA, allowing them to integrate into the host cell's DNA and replicate. RT inhibitors work by inhibiting the activity of reverse transcriptase, preventing the virus from replicating and reducing the viral load in the body. There are two types of RT inhibitors: nucleoside/nucleotide RT inhibitors (NRTIs) and non-nucleoside RT inhibitors (NNRTIs). NRTIs resemble the building blocks of DNA and when incorporated into the viral DNA chain, they terminate its elongation. NNRTIs bind directly to reverse transcriptase, causing a conformational change that inhibits its activity. By targeting reverse transcriptase, RT inhibitors play a crucial role in suppressing viral replication and slowing down the progression of viral diseases like HIV/AIDS.
Drug Target R&D Trends for tenofovir alafenamide fumarate
Respiratory therapy (RT) plays a crucial role in the human body by focusing on the prevention, diagnosis, and treatment of respiratory disorders. RT professionals are trained to assess and manage patients with breathing difficulties, such as asthma, chronic obstructive pulmonary disease (COPD), and respiratory infections. They utilize various techniques and equipment to improve lung function, including administering medications, oxygen therapy, and mechanical ventilation. RT also involves educating patients on proper breathing techniques, lifestyle modifications, and disease management strategies. By ensuring optimal respiratory health, RT helps individuals maintain a higher quality of life and reduces the risk of complications associated with respiratory conditions.
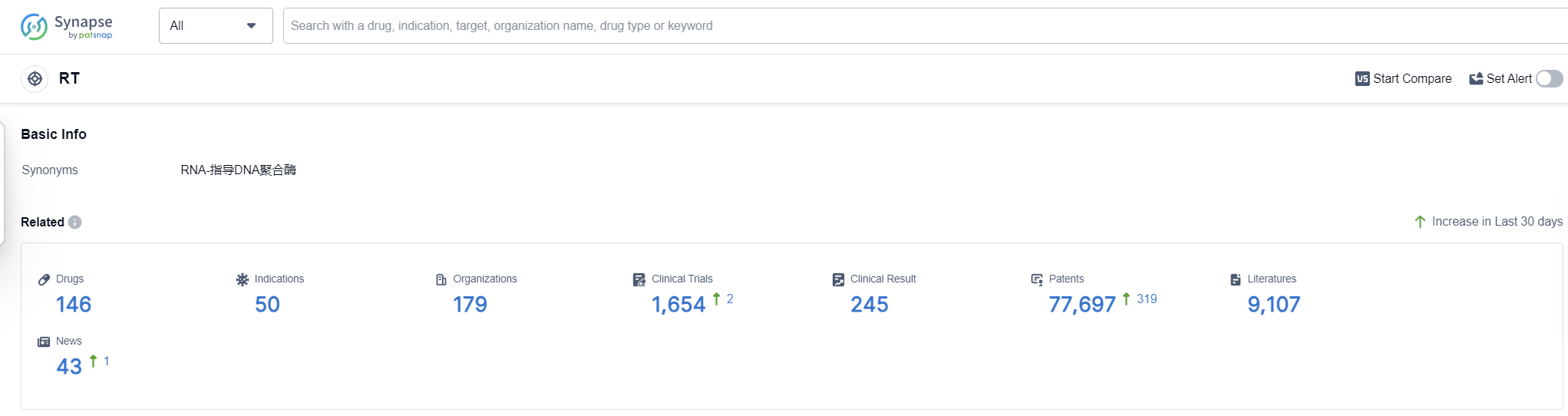
According to Patsnap Synapse, as of 16 Sep 2023, there are a total of 146 RT drugs worldwide, from 179 organizations, covering 50 indications, and conducting 1654 clinical trials.
Based on the analysis of the data, the current competitive landscape of target RT in the pharmaceutical industry is characterized by the presence of several companies that are growing rapidly, with Gilead Sciences, Inc. leading in terms of the highest stage of development. The indications for drugs under this target are diverse, with a focus on HIV Infections, Hepatitis B, and Acquired Immunodeficiency Syndrome.
The most rapidly progressing drug types are small molecule drugs and synthetic peptides, indicating intense competition and innovation in these areas. China has shown significant progress in the development of drugs under this target, along with other countries such as the United States, European Union, and Japan.
To ensure future development and competitiveness in the target RT, companies should continue their R&D efforts, especially in the areas of small molecule drugs and synthetic peptides. Collaboration between pharmaceutical companies and research institutions in China and other countries can further accelerate the development of drugs for target RT. Continuous monitoring of the competitive landscape and regulatory environment will be crucial for success in this market.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Tenofovir Alafenamide Fumarate is a small molecule drug developed by Gilead Sciences, Inc. It targets the reverse transcriptase enzyme and has been approved for use in the treatment of immune system diseases, infectious diseases, urogenital diseases, and digestive system disorders. The drug has shown efficacy in addressing virus diseases, chronic hepatitis B, liver diseases, and HIV infections. Its approval in multiple countries, including the United States, highlights its potential to provide therapeutic benefits to patients globally. The drug's regulatory status as a "Special Review Project" suggests that it may have undergone additional evaluation due to specific factors.