Analysis on the Clinical Research Progress of Aromatase Inhibitor
Aromatase is an enzyme that plays a crucial role in the human body by converting androgens (male hormones) into estrogens (female hormones). It is primarily found in various tissues, including the ovaries, testes, adrenal glands, and fat cells. Aromatase is responsible for the synthesis of estrogen, which is essential for the development and functioning of reproductive organs, bone health, and regulation of the menstrual cycle in females. In males, Aromatase helps maintain a balance between androgens and estrogens, contributing to normal sexual development and fertility. The inhibition or modulation of Aromatase activity is a target for pharmaceutical interventions in conditions such as breast cancer, endometriosis, and hormone-related disorders.
Aromatase Competitive Landscape
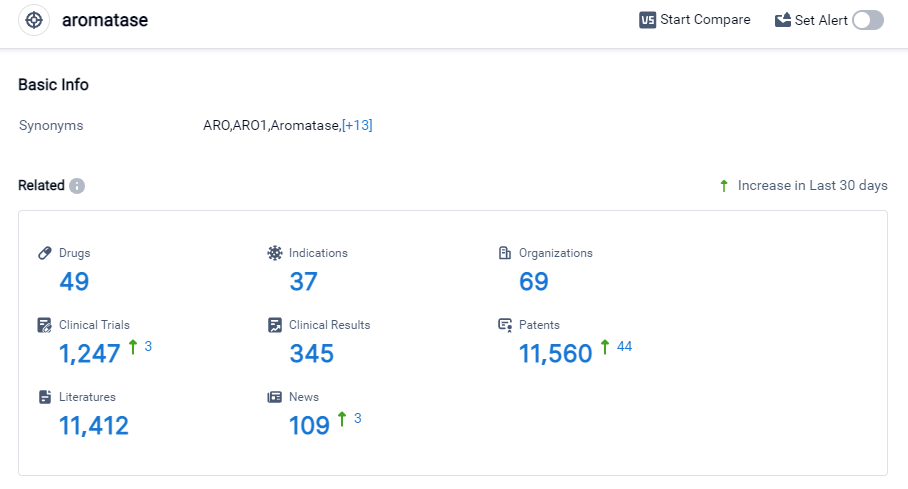
According to Patsnap Synapse, as of 27 Sep 2023, there are a total of 49 Aromatase drugs worldwide, from 69 organizations, covering 37 indications, and conducting 1247 clinical trials.
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs , indications, organizations, clinical trials, clinical results, and drug patents related to this target.
The current competitive landscape of target Aromatase is characterized by the involvement of multiple companies, with Novartis AG, Pfizer Inc., and AstraZeneca PLC leading in terms of the highest stage of development. These companies have made significant progress in R&D, with approved drugs targeting various indications.
The most common indication for drugs targeting Aromatase is breast cancer, followed by other indications such as prostatic hyperplasia, infertility, and pituitary ACTH hypersecretion. However, there is still a need for further research and development in many indications.
Small molecule drugs are progressing most rapidly under the current target Aromatase, indicating intense competition in this area. Other drug types, such as chemical drugs, diagnostic radiopharmaceuticals, small interfering RNA, and unknown drugs, are also being developed but have fewer representatives.
The United States, China, the European Union, and Japan are the countries/locations with the highest number of approved drugs targeting Aromatase. China, in particular, has shown significant progress in this area. Other countries are also actively involved in the research and development of drugs targeting Aromatase, but to a lesser extent.
Overall, the target Aromatase market is competitive, with multiple companies and countries involved in the development of drugs. Further research and development are needed to explore new indications and drug types, and to continue advancing the field of Aromatase inhibition.
Key drug: Letrozole
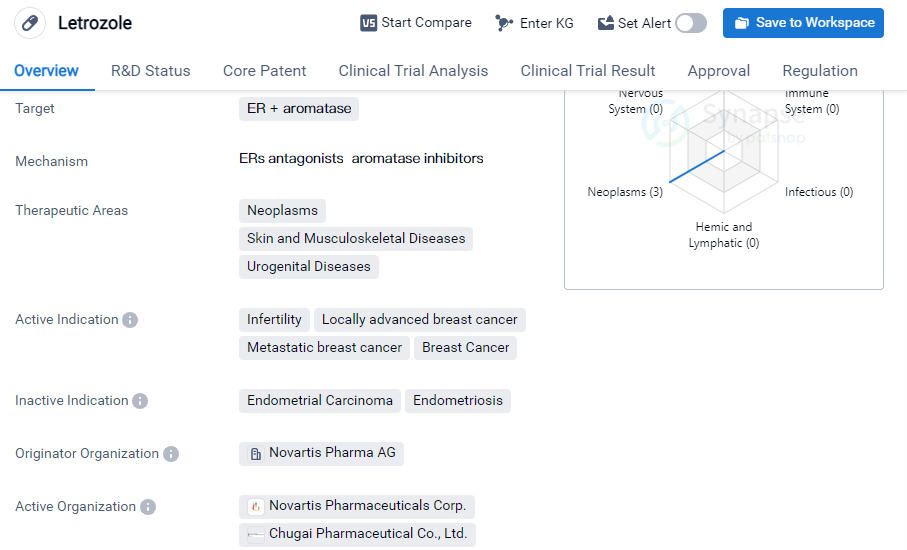
Letrozole is a small molecule drug that is primarily used in the treatment of various neoplasms, skin and musculoskeletal diseases, and urogenital diseases. It specifically targets estrogen receptor-positive (ER+) Aromatase, making it effective in managing conditions related to hormone imbalances.
The drug has been approved for several indications, including infertility, locally advanced breast cancer, metastatic breast cancer, and breast cancer in general. Letrozole is known to be particularly effective in treating breast cancer cases where the tumor is hormone receptor-positive. It works by inhibiting the production of estrogen, which is known to fuel the growth of hormone receptor-positive breast cancer cells.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Letrozole was first approved globally in July 1996 and was initially introduced in Switzerland and Sweden. The drug has since gained approval in various other countries, including China. Its approval in multiple countries indicates its widespread recognition and acceptance as an effective treatment option.
The originator organization of Letrozole is Novartis Pharma AG, a renowned pharmaceutical company known for its contributions to the development of innovative drugs. Novartis Pharma AG has played a significant role in bringing Letrozole to the market and ensuring its availability to patients worldwide.
In terms of regulatory status, Letrozole has undergone priority review and accelerated approval processes. These regulatory pathways are designed to expedite the approval of drugs that address unmet medical needs or provide significant therapeutic benefits. The utilization of these regulatory pathways suggests that Letrozole has demonstrated promising results in clinical trials and has the potential to address critical medical conditions.
In summary, Letrozole is a small molecule drug that targets ER+ Aromatase and is primarily used in the treatment of neoplasms, skin and musculoskeletal diseases, and urogenital diseases. It has been approved for various indications, including infertility and breast cancer, and is recognized globally as an effective treatment option. Letrozole was first approved in 1996 and is manufactured by Novartis Pharma AG. Its regulatory status includes priority review and accelerated approval, indicating its potential to address unmet medical needs.