Deep Scientific Insights on Ceritinib's R&D Progress, Mechanism of Action, and Drug Target
Ceritinib's R&D Progress
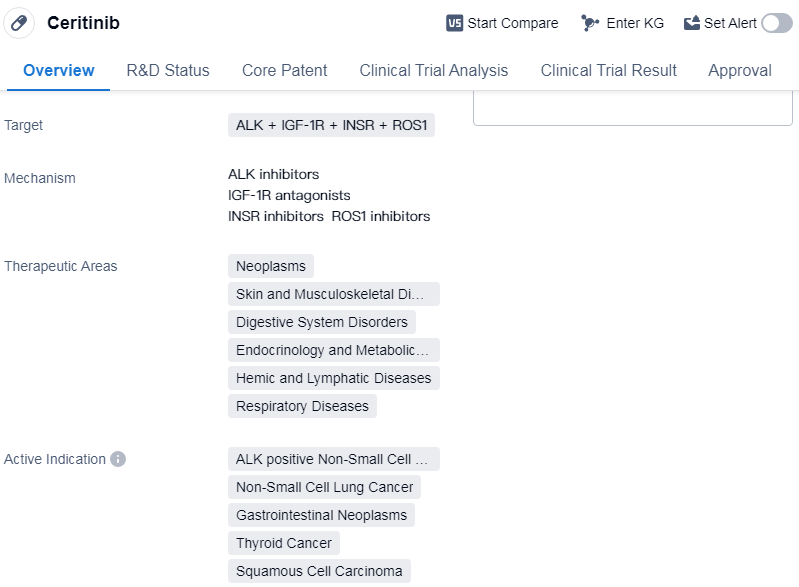
Ceritinib is a small molecule drug that is primarily used in the treatment of various types of neoplasms, skin and musculoskeletal diseases, digestive system disorders, endocrinology and metabolic diseases, hemic and lymphatic diseases, and respiratory diseases. It targets multiple proteins including ALK, IGF-1R, INSR, and ROS1.
Ceritinib is indicated for the treatment of ALK-positive non-small cell lung cancer (NSCLC), NSCLC, gastrointestinal neoplasms, thyroid cancer, squamous cell carcinoma, solid tumors, hematologic neoplasms, and melanoma. It has been approved for use in the United States since April 2014.
Ceritinib was developed by Novartis Pharma AG, a leading pharmaceutical company. It has reached the highest phase of development, which is approved globally. This indicates that it has successfully completed clinical trials and has been deemed safe and effective for use in patients.
The drug has undergone various regulatory processes, including priority review, accelerated approval, breakthrough therapy designation, and orphan drug designation. These regulatory designations highlight the potential significance of ceritinib in addressing unmet medical needs and its potential to provide significant benefits to patients.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Ceritinib: ALK inhibitors, IGF-1R antagonists, INSR inhibitors and ROS1 inhibitors
ALK inhibitors are a type of medication that specifically target and inhibit the activity of anaplastic lymphoma kinase (ALK), a protein that is involved in cell growth and division. These inhibitors are commonly used in the treatment of certain types of cancer, such as NSCLC, that have genetic alterations or mutations in the ALK gene. By blocking the activity of ALK, these inhibitors can help to slow down or stop the growth of cancer cells.
IGF-1R antagonists are drugs that act as antagonists, meaning they block the activity of insulin-like growth factor-1 receptor (IGF-1R). IGF-1R is a cell surface receptor that plays a role in cell growth, proliferation, and survival. By inhibiting the activity of IGF-1R, these antagonists can interfere with the signaling pathways that promote cancer cell growth and potentially inhibit tumor growth.
INSR inhibitors are medications that target and inhibit the activity of insulin receptor (INSR). INSR is a receptor protein that plays a crucial role in regulating glucose metabolism and cell growth. Inhibiting the activity of INSR can help to regulate blood sugar levels and may have potential therapeutic applications in conditions such as diabetes and certain types of cancer.
ROS1 inhibitors are drugs that specifically target and inhibit the activity of ROS1, a receptor tyrosine kinase that is involved in cell growth and proliferation. ROS1 gene rearrangements or mutations can occur in certain types of cancer, particularly NSCLC. By inhibiting the activity of ROS1, these inhibitors can help to slow down or stop the growth of cancer cells with ROS1 alterations.
Overall, these drugs belong to different classes of targeted therapies that aim to interfere with specific molecules or pathways involved in disease progression, particularly in cancer. They have shown promising results in clinical trials and are being used in the treatment of specific types of cancer where these molecular targets are known to be altered.
Drug Target R&D Trends for Ceritinib
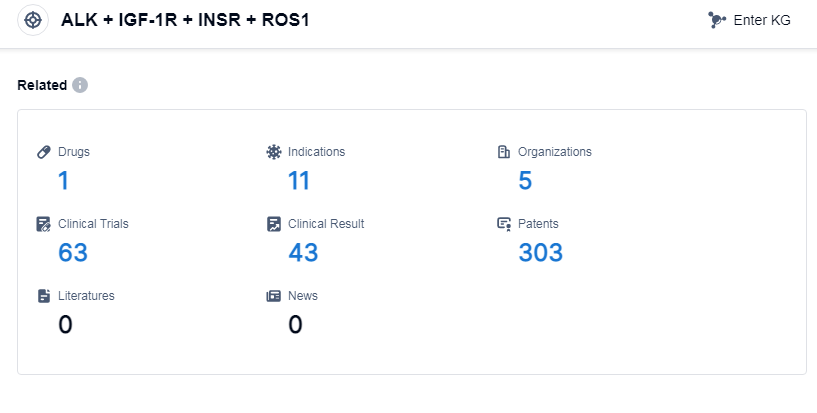
According to PatSnap Synapse, as of 8 Sep 2023, there are a total of 1 ALK, IGF-1R, INSR and ROS1 drugs worldwide, from 5 organizations, covering 11 indications, and conducting 63 clinical trials.
The analysis of the target ALK, IGF-1R, INSR and ROS1 reveals a competitive landscape with active research and development from various companies, including Novartis AG. The target has approved for drugs with indications such as ALK-positive NSCLC, solid tumors, hematologic neoplasms, thyroid cancer, melanoma, etc. Small molecule drugs dominate the highest phase under this target, indicating a focus on innovation. The presence of biosimilars in the inactive phase suggests potential competition. Additionally, countries/locations such as the European Union, United States, South Korea, China, and others are actively developing drugs under this target. Further analysis is required to understand the specific drugs, R&D progress, and impact of these developments on the pharmaceutical industry.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, ceritinib is a small molecule drug developed by Novartis Pharma AG. It targets multiple proteins and is indicated for the treatment of various types of neoplasms and other diseases. It has been approved for use in the United States since 2014 and has also received approval in China. The drug has undergone rigorous regulatory processes, indicating its potential to address unmet medical needs and provide significant benefits to patients.