Deep Scientific Insights on Naloxegol Oxalate's R&D Progress, Mechanism of Action, and Drug Target
Naloxegol Oxalate's R&D Progress
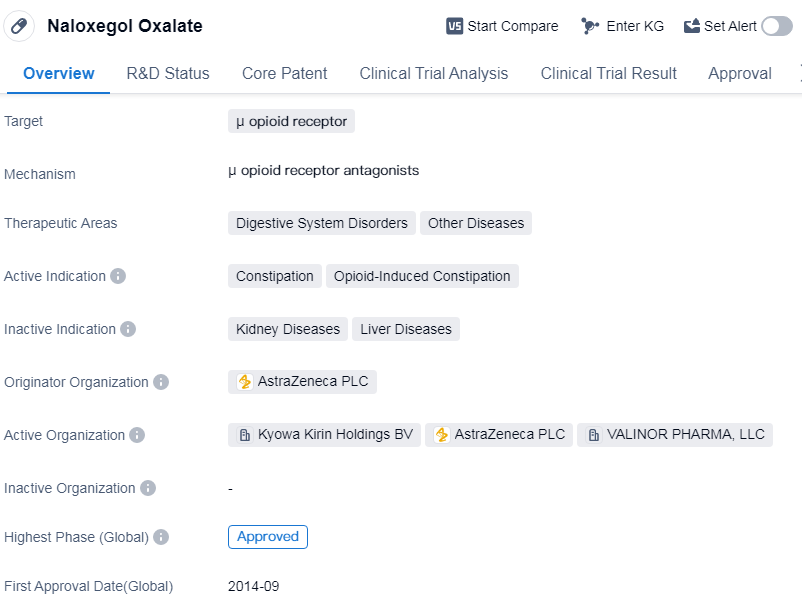
Naloxegol Oxalate is a small molecule drug that targets the μ opioid receptor. It is primarily used in the treatment of digestive system disorders and other diseases. The drug is specifically indicated for the management of constipation, particularly opioid-induced constipation.
The originator organization of Naloxegol Oxalate is AstraZeneca PLC, a multinational pharmaceutical company. The drug has reached the highest phase of development, which is approval. It received its first approval in September 2014 in the United States.
Naloxegol Oxalate is a significant development in the field of biomedicine as it addresses a common side effect of opioid use, which is constipation. Opioids are commonly prescribed for pain management, but they can cause constipation due to their effects on the μ opioid receptor. By targeting this receptor, Naloxegol Oxalate helps to alleviate constipation in patients taking opioids.
The approval of Naloxegol Oxalate in the United States in 2014 marked an important milestone in the treatment of opioid-induced constipation. It provided healthcare professionals with a new option to manage this side effect and improve the quality of life for patients. The drug's approval demonstrates its safety and efficacy in clinical trials, leading to its acceptance by regulatory authorities.
The therapeutic areas of digestive system disorders and other diseases highlight the potential applications of Naloxegol Oxalate beyond constipation. While the specific diseases within these areas are not mentioned, it suggests that the drug may have broader therapeutic uses. Further research and clinical trials may explore these potential indications.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Naloxegol Oxalate: μ opioid receptor antagonists
From a biomedical perspective, μ opioid receptor antagonists are a type of drugs that block or inhibit the activity of the μ opioid receptors in the body. The μ opioid receptors are a subtype of opioid receptors found in the central nervous system and are involved in mediating the effects of opioids, such as pain relief, sedation, and euphoria. By antagonizing these receptors, μ opioid receptor antagonists can counteract the effects of opioids and are commonly used for the treatment of opioid overdose or addiction. These drugs can help reverse the respiratory depression caused by opioid overdose and reduce the rewarding effects of opioids, thereby aiding in the management of opioid dependence. Examples of μ opioid receptor antagonists include naloxone and naltrexone.
Drug Target R&D Trends for Naloxegol Oxalate
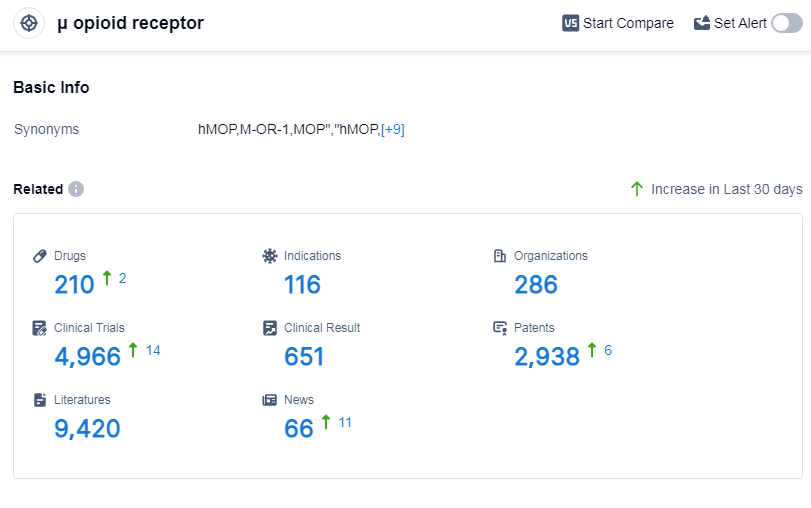
According to Patsnap Synapse, as of 14 Sep 2023, there are a total of 210 μ opioid receptor drugs worldwide, from 286 organizations, covering 116 indications, and conducting 4966 clinical trials.
The analysis of the target μ opioid receptor reveals a competitive landscape with multiple companies making progress in drug development. Johnson & Johnson, Collegium Pharmaceutical, Inc., and Wuhan Dangdai Technology Industry Group Co. Ltd. are among the companies growing fastest under this target. The highest stage of development is the approved phase, indicating successful R&D efforts.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Naloxegol Oxalate is a small molecule drug developed by AstraZeneca PLC that targets the μ opioid receptor. It is approved for the treatment of constipation, particularly opioid-induced constipation. The drug's approval in the United States in 2014 signifies its effectiveness and safety in clinical trials. Its therapeutic areas of digestive system disorders and other diseases indicate potential applications beyond constipation.