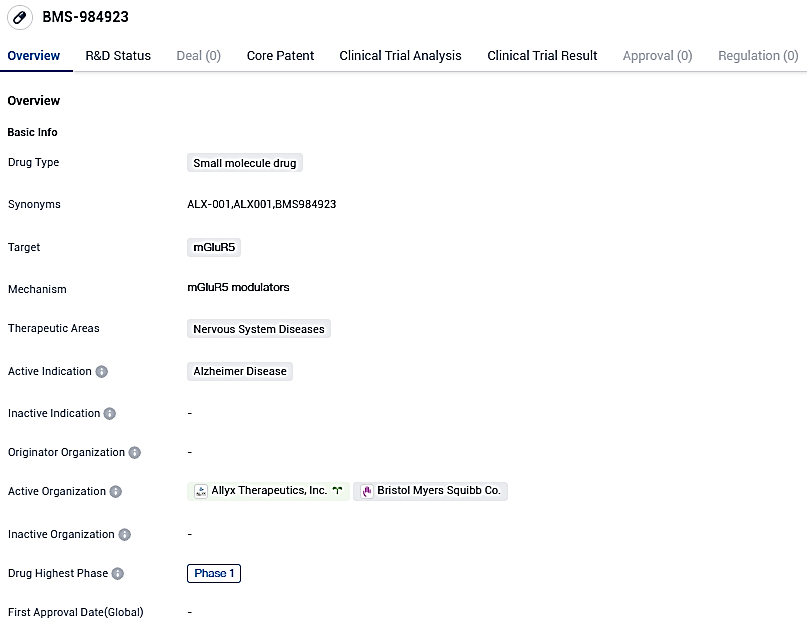
Allyx Therapeutics' primary compound, ALX-001, demonstrates safety and toleration through dosage testing and successfully engages with the brains of healthy seniors
On October 27, 2023, at the 16th Clinical Trials on Alzheimer's Disease convention held in Boston, Allyx Therapeutics, a clinical-phase biotechnology firm currently working on creating ALX-001- an orally administered, first-of-its-kind, highly selective, synapse-oriented, disease-altering treatment for neurodegenerative conditions, disclosed the findings of their Phase 1a single ascending dose study.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The research indicated that each dosage of ALX-001 exhibited good tolerance, even at higher concentrations capable of achieving a comprehensive engagement with brain targets, thus encouraging the firm's decision to proceed with clinical development in treating Alzheimer's disease.
In this preliminary 1a open-label trial, the safety, acceptability, pharmacokinetics, and brain receptor occupancy of rising doses of orally-given ALX-001 was assessed. A single ascending dose was given to 36 mentally healthy adults aged between 50 to 80 years old. The results related to safety were also quite reassuring.
Each dosage of ALX-001 was accepted well with no major harmful events recorded. The Principal Researcher, Adam Mecca, M.D., Ph.D. of Yale School of Medicine Alzheimer's Disease Research Unit presented the data. The research was conducted at Yale and obtained funding from the National Institutes of Health and Alzheimer’s Association.
"The extraordinary way that ALX-001 operates on mGluR5 has the potential to be an innovative approach to preserving and safeguarding synaptic connections for those struggling with neurodegenerative disorders," shared Tim Siegert, Ph.D., the chief operating officer, and co-founding member of Allyx Therapeutics. "We are incredibly excited about the clinical community's reaction to our preliminary clinical data as we strive to introduce a pioneering oral treatment for individuals living with Alzheimer's disease."
ALX-001 continues to show potential in ongoing investigations based on twelve years of study. Clinical research of ALX-001 in Alzheimer's disease is making progress, with a phase 1 multiple ascending dose trial in healthy volunteers projected to conclude in Q4 2023.
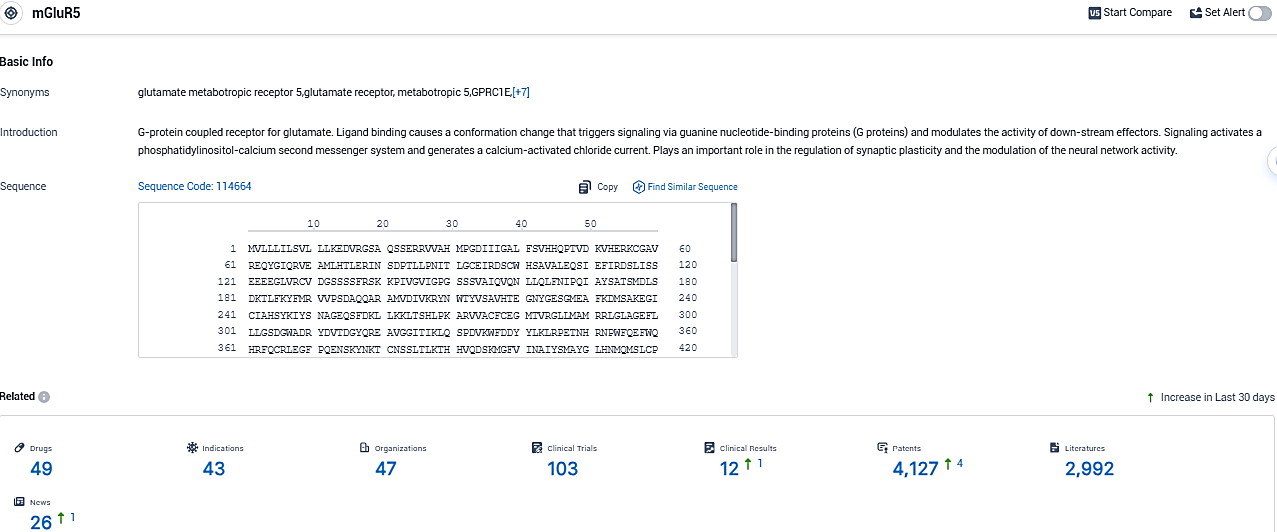
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 2, 2023, there are 49 investigational drugs for the mGluR5 target, including 43 indications, 47 R&D institutions involved, with related clinical trials reaching 103, and as many as 4127 patents.
ALX-001 is an unobtrusive allosteric modulator specific to mGluR5 and pioneers a new class of compounds that selectively deter the harmful activation of the receptor. It concurrently maintains the standard physiological glutamate signaling crucial for cognition. Consequently, ALX-001 presents a broad treatment range capable of saturating receptors without the on-target toxicity seen in negative allosteric modulators. Allyx Therapeutics has procured an exclusive global license for employing ALX-001 from Bristol Myers Squibb and Yale University.