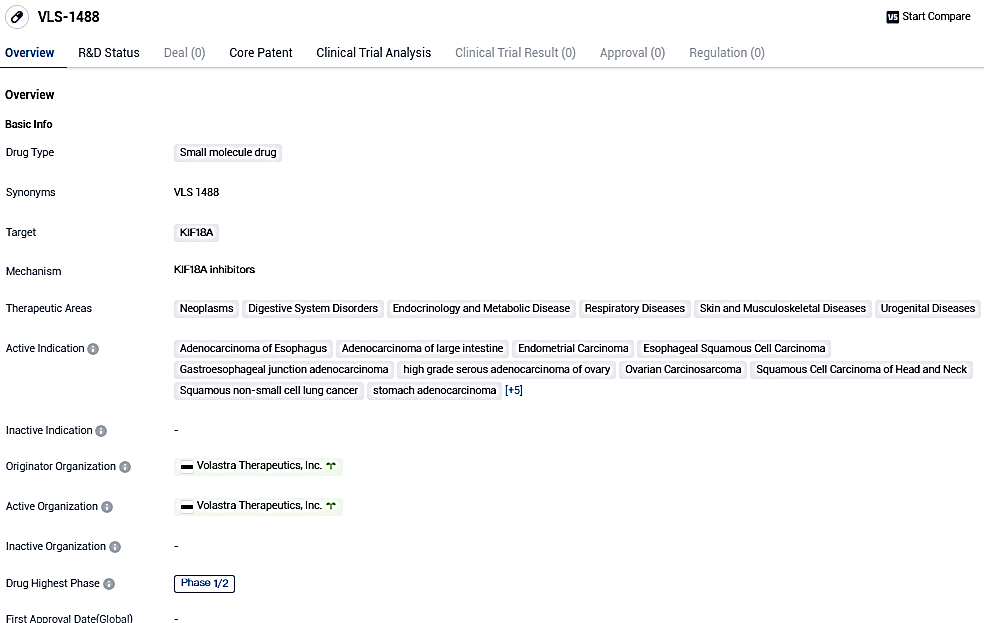
Volastra Therapeutics reports that the initial patient has been treated in the Phase I/II Clinical Study of VLS-1488, a unique KIF18A inhibitor in its innovative compound portfolio
Volastra Therapeutics, a biotech company specialized in discovering and developing cancer treatments, has reported the initiation of dosing for the inaugural participant in a Phase I/II clinical trial of VLS-1488. The firm's forward-thinking clinical portfolio, made up of VLS-1488 and sovilnesib, consists of unique KIF18A inhibitors specifically developed to cure solid tumors exhibiting high amounts of chromosomal instability.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
The Phase I/II study is scrutinizing the safety, tolerability, and initial effectiveness of VLS-1488 for patients grappling with advanced tumors. The study serves as a crucial milestone in Volastra's mission to offer aid to patients battling these complex cancers.
"Two years ago, our team embarked on a journey to comprehend the biology of chromosomal instability and to uncover new therapeutic approaches”, shared Charles Hugh-Jones M.D., FRCP, CEO at Volastra. “The pioneering work leading to this research could potentially establish the first-ever CIN-focused therapy.”
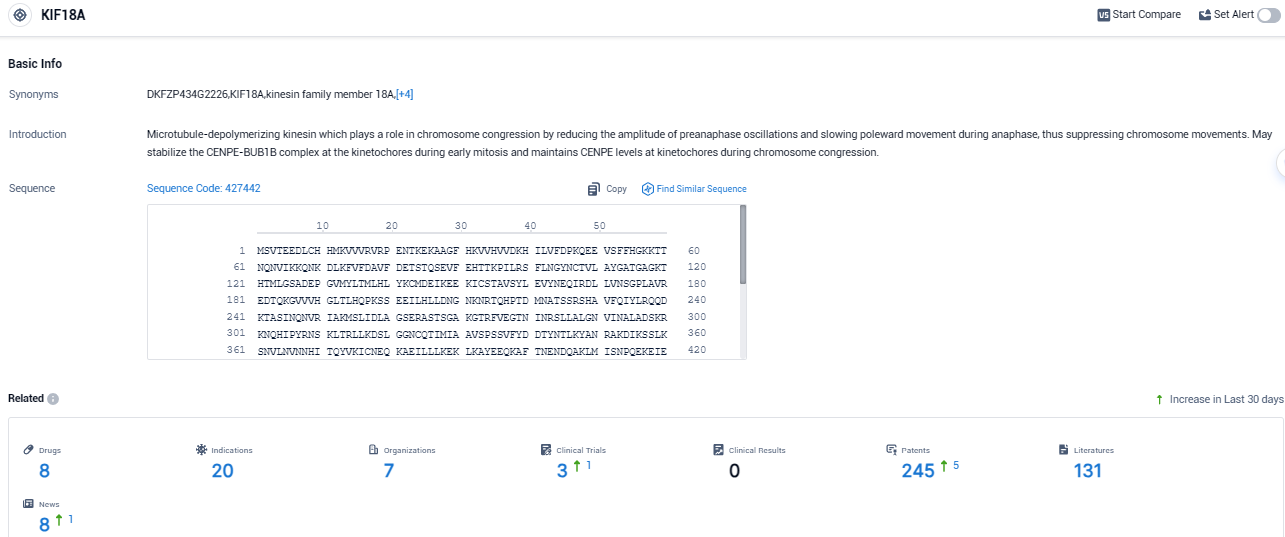
KIF18A is a protein which is essential for many tumor cells to split and grow proficiently. This protein is not indispensable for normal healthy cells to divide, hence allowing the targeted eradication of cancer cells. Volastra plans to employ a range of exploratory biomarkers to gauge CIN, amongst which includes AI-based tissue imaging developed in collaboration with Microsoft.
Dr. Pat LoRusso, D.O., Head of the Phase I Clinical Trial Unit at Yale noted, "Addressing chromosomal instability presents a significant prospect for cancer treatment. Blockading KIF18A has demonstrated remarkable data in pre-clinical models with high CIN levels. We eagerly anticipate contributing to the clinical study of this hopeful molecule.”
👇Please click on the picture link below for free registration or login directly if you have freemium accounts, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target.
According to the data provided by the Synapse Database, As of November 2, 2023, there are 8 investigational drugs for the KIF18A target, including 20 indications, 7 R&D institutions involved, with related clinical trials reaching 3, and as many as 245 patents.
VLS-1488 has displayed possible curative advantages in multiple therapeutic fields, specifically in managing diverse forms of cancer. Presently in the Phase 1/2 clinical development stage, additional study is required to thoroughly comprehend its mode of operation and assess its safety and effectiveness.