Deep Scientific Insights on Neostigmine Methylsulfate's R&D Progress
Neostigmine Methylsulfate's R&D Progress
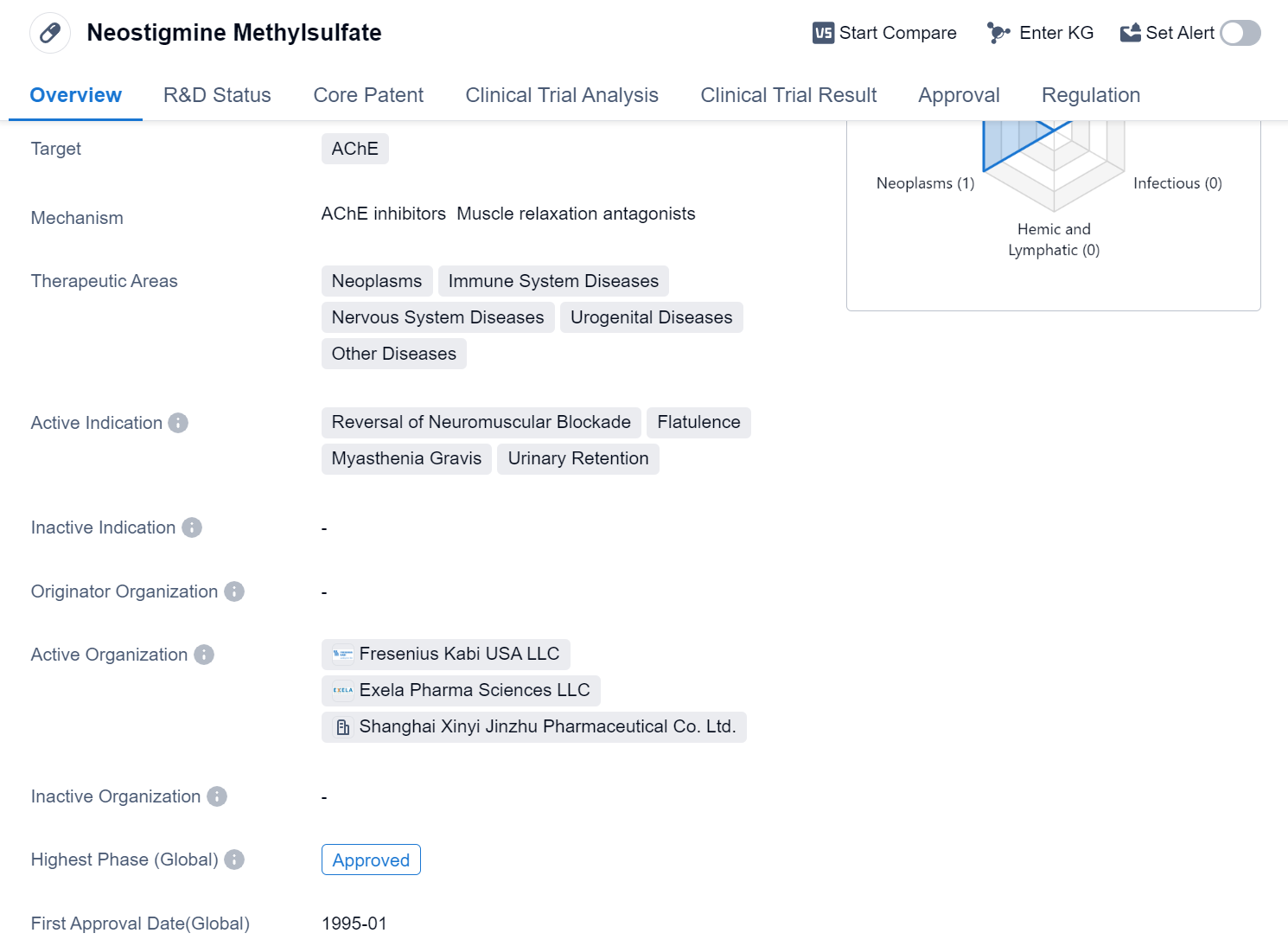
Neostigmine Methylsulfate is a small molecule drug that primarily targets the enzyme acetylcholinesterase (AChE). It has been approved for use in various therapeutic areas, including neoplasms, immune system diseases, nervous system diseases, urogenital diseases, and other diseases. The drug is indicated for the reversal of neuromuscular blockade, flatulence, myasthenia gravis, and urinary retention.
Neostigmine Methylsulfate has successfully completed the highest phase of clinical trials and has received approval globally. Its first approval date was in January 1995, with China being the first country to approve its use. The drug is regulated as an orphan drug, indicating that it is intended to treat rare diseases or conditions.
As a small molecule drug, Neostigmine Methylsulfate is designed to interact with specific targets in the body, in this case, AChE. By inhibiting the activity of this enzyme, the drug can help reverse neuromuscular blockade, a condition characterized by the inability to move or control muscles. It is also used to alleviate flatulence, a common symptom of gastrointestinal disorders, and to manage myasthenia gravis, an autoimmune neuromuscular disease.
Additionally, Neostigmine Methylsulfate is indicated for the treatment of urinary retention, a condition where the bladder is unable to empty completely. By stimulating the muscles responsible for bladder contraction, the drug can help improve urinary flow.
Since its first approval in 1995, the drug has likely been widely used in clinical settings to provide relief for patients suffering from neuromuscular blockade, flatulence, myasthenia gravis, and urinary retention.
👇Please click on the image below to directly access the latest data (R&D Status | Core Patent | Clinical Trial | Approval status in Global countries) of this drug.
Mechanism of Action for Neostigmine Methylsulfate: AChE inhibitors & Muscle relaxation antagonists
AChE inhibitors, also known as acetylcholinesterase inhibitors, are a class of drugs that inhibit the activity of the enzyme acetylcholinesterase. Acetylcholinesterase is responsible for breaking down the neurotransmitter acetylcholine, which plays a crucial role in transmitting signals between nerve cells. By inhibiting acetylcholinesterase, AChE inhibitors increase the levels of acetylcholine in the brain, leading to enhanced cholinergic neurotransmission.
From a biomedical perspective, AChE inhibitors are commonly used in the treatment of various conditions, including Alzheimer's disease, myasthenia gravis, and Parkinson's disease. In Alzheimer's disease, the progressive loss of cholinergic neurons leads to a decrease in acetylcholine levels, contributing to cognitive decline. AChE inhibitors help to increase acetylcholine levels and improve cognitive function in these patients.
Muscle relaxation antagonists, on the other hand, are drugs that act as antagonists at the neuromuscular junction, blocking the action of acetylcholine on muscle cells. This leads to muscle relaxation and paralysis, which can be beneficial during surgical procedures or when mechanical ventilation is required. These drugs are commonly used in anesthesia to induce muscle relaxation and facilitate intubation.
In summary, AChE inhibitors are drugs that increase acetylcholine levels by inhibiting the enzyme acetylcholinesterase, while muscle relaxation antagonists block the action of acetylcholine to induce muscle relaxation.
Drug Target R&D Trends for Neostigmine Methylsulfate
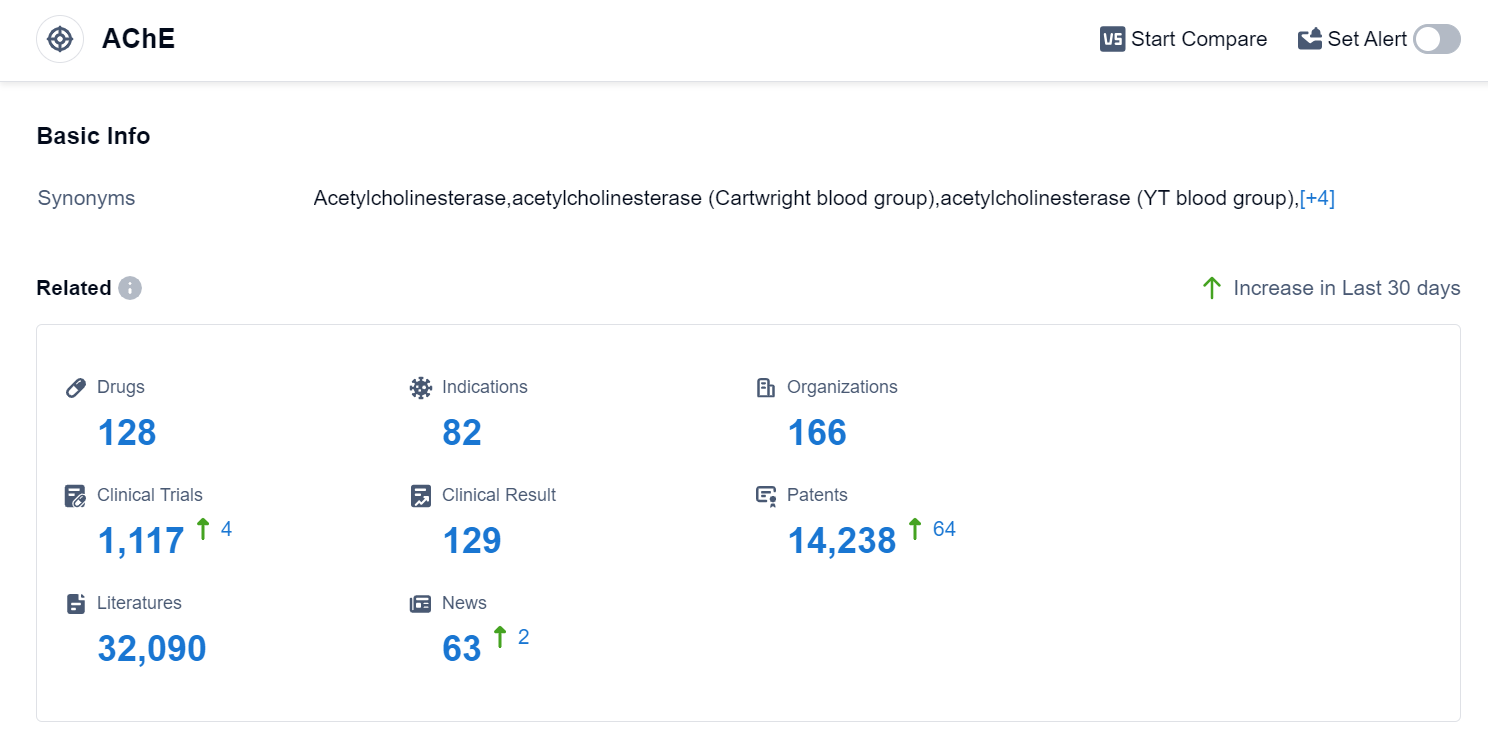
According to Patsnap Synapse, as of 10 Sep 2023, there are a total of 128 AChE drugs worldwide, from 166 organizations, covering 82 indications, and conducting 1117 clinical trials.
The analysis of the target AChE reveals a competitive landscape with multiple companies actively involved in R&D. AbbVie, Inc., Shanghai Pharmaceuticals Holding Co., Ltd., Pfizer Inc., Zeria Pharmaceutical Co., Ltd., and Eisai Co., Ltd. are among the companies growing fastest in this area. The approved drugs for the target AChE cover indications such as Myasthenia Gravis, Alzheimer Disease, Poisoning, Glaucoma, and more. Small molecule drugs dominate the drug types progressing rapidly under the target AChE, indicating intense competition. China, the United States, Japan, and the European Union are the countries/locations developing fastest under the target AChE, with China showing significant progress. Overall, the target AChE presents a promising area for pharmaceutical development, with potential applications in various neurological and gastrointestinal disorders.
👇Please click on the picture link below for free registration or log in directly if you have a freemium account, you can browse the latest research progress on drugs, indications, organizations, clinical trials, clinical results, and drug patents related to this target
Conclusion
In summary, Neostigmine Methylsulfate is a small molecule drug that targets AChE and has been approved for various therapeutic areas. Its indications include the reversal of neuromuscular blockade, flatulence, myasthenia gravis, and urinary retention. With its global approval, as well as its orphan drug status, Neostigmine Methylsulfate has demonstrated its potential in addressing specific medical needs.